Antibody fusion proteins: anti-CD22 recombinant immunotoxin moxetumomab pasudotox
- PMID: 22003067
- PMCID: PMC3201735
- DOI: 10.1158/1078-0432.CCR-11-0487
Antibody fusion proteins: anti-CD22 recombinant immunotoxin moxetumomab pasudotox
Abstract
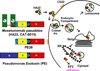
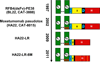
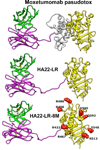
Recombinant immunotoxins are fusion proteins that contain the cytotoxic portion of a protein toxin fused to the Fv portion of an antibody. The Fv binds to an antigen on a target cell and brings the toxin into the cell interior, where it arrests protein synthesis and initiates the apoptotic cascade. Moxetumomab pasudotox, previously called HA22 or CAT-8015, is a recombinant immunotoxin composed of the Fv fragment of an anti-CD22 monoclonal antibody fused to a 38-kDa fragment of Pseudomonas exotoxin A, called PE38. Moxetumomab pasudotox is an improved, more active form of a predecessor recombinant immunotoxin, BL22 (also called CAT-3888), which produced complete remission in relapsed/refractory hairy cell leukemia (HCL), but it had a <20% response rate in chronic lymphocytic leukemia (CLL) and acute lymphoblastic leukemia (ALL), diseases in which the leukemic cells contain much lower numbers of CD22 target sites. Compared with BL22, moxetumomab pasudotox is up to 50-fold more active on lymphoma cell lines and leukemic cells from patients with CLL and HCL. A phase I trial was recently completed in HCL patients, who achieved response rates similar to those obtained with BL22 but without dose-limiting toxicity. In addition to further testing in HCL, moxetumomab pasudotox is being evaluated in phase I trials in patients with CLL, B-cell lymphomas, and childhood ALL. Moreover, protein engineering is being used to increase its activity, decrease nonspecific side effects, and remove B-cell epitopes.
©2011 AACR.
Figures
Similar articles
-
Moxetumomab Pasudotox: First Global Approval.Drugs. 2018 Nov;78(16):1763-1767. doi: 10.1007/s40265-018-1000-9. Drugs. 2018. PMID: 30357593 Free PMC article. Review.
-
Immunoconjugates in the management of hairy cell leukemia.Best Pract Res Clin Haematol. 2015 Dec;28(4):236-45. doi: 10.1016/j.beha.2015.09.003. Epub 2015 Oct 9. Best Pract Res Clin Haematol. 2015. PMID: 26614902 Free PMC article. Review.
-
Moxetumomab pasudotox for the treatment of hairy cell leukemia.Expert Opin Biol Ther. 2019 Jun;19(6):501-508. doi: 10.1080/14712598.2019.1614558. Epub 2019 May 10. Expert Opin Biol Ther. 2019. PMID: 31045462 Review.
-
Phase 1 study of the anti-CD22 immunotoxin moxetumomab pasudotox for childhood acute lymphoblastic leukemia.Blood. 2017 Oct 5;130(14):1620-1627. doi: 10.1182/blood-2017-02-749101. Epub 2017 Aug 9. Blood. 2017. PMID: 28983018 Free PMC article. Clinical Trial.
-
Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia.J Clin Oncol. 2012 May 20;30(15):1822-8. doi: 10.1200/JCO.2011.38.1756. Epub 2012 Feb 21. J Clin Oncol. 2012. PMID: 22355053 Free PMC article. Clinical Trial.
Cited by
-
ADAM17-overexpressing breast cancer cells selectively targeted by antibody-toxin conjugates.Cancer Immunol Immunother. 2013 Mar;62(3):411-21. doi: 10.1007/s00262-012-1346-x. Epub 2012 Sep 1. Cancer Immunol Immunother. 2013. PMID: 22940887 Free PMC article.
-
Contextualizing the Use of Moxetumomab Pasudotox in the Treatment of Relapsed or Refractory Hairy Cell Leukemia.Oncologist. 2020 Jan;25(1):e170-e177. doi: 10.1634/theoncologist.2019-0370. Epub 2019 Oct 18. Oncologist. 2020. PMID: 31628266 Free PMC article.
-
Current Status of Novel Agents for the Treatment of B Cell Malignancies: What's Coming Next?Cancers (Basel). 2022 Dec 7;14(24):6026. doi: 10.3390/cancers14246026. Cancers (Basel). 2022. PMID: 36551511 Free PMC article. Review.
-
HER2-Specific Targeted Toxin DARPin-LoPE: Immunogenicity and Antitumor Effect on Intraperitoneal Ovarian Cancer Xenograft Model.Int J Mol Sci. 2019 May 15;20(10):2399. doi: 10.3390/ijms20102399. Int J Mol Sci. 2019. PMID: 31096563 Free PMC article.
-
Plant/Bacterial Virus-Based Drug Discovery, Drug Delivery, and Therapeutics.Pharmaceutics. 2019 May 3;11(5):211. doi: 10.3390/pharmaceutics11050211. Pharmaceutics. 2019. PMID: 31058814 Free PMC article. Review.
References
-
- Witzig TE. Radioimmunotherapy for B-cell non-Hodgkin lymphoma. Best Pract Res Clin Haematol. 2006;19:655–668. - PubMed
-
- Steiner M, Neri D. Antibody-radionuclide conjugates for cancer therapy: historical considerations and new trends. Clin Cancer Res. 2011;17:xx. - PubMed
-
- Teicher BA, Chari RVJ. Antibody-drug conjugate therapeutics: challenges and potential. Clin Cancer Res. 2011;17:xx. - PubMed
-
- LoRusso PM, Weiss D, Guardino E, Girish S, Sliwkowski MX. Trastuzumab emtansine: a unique antibody-drug conjugate in development for human epidermal growth factor receptor 2–positive cancer. Clin Cancer Res. 2011;17:xx. 2011. - PubMed
-
- Katz J, Janik JE, Younes A. Brentuximab Vedotin - SGN-35. Clin Cancer Res. 2011;17:XX. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

