Actin interacting protein1 and actin depolymerizing factor drive rapid actin dynamics in Physcomitrella patens
- PMID: 22003077
- PMCID: PMC3229144
- DOI: 10.1105/tpc.111.090753
Actin interacting protein1 and actin depolymerizing factor drive rapid actin dynamics in Physcomitrella patens
Abstract
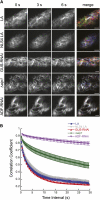
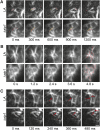
The remodeling of actin networks is required for a variety of cellular processes in eukaryotes. In plants, several actin binding proteins have been implicated in remodeling cortical actin filaments (F-actin). However, the extent to which these proteins support F-actin dynamics in planta has not been tested. Using reverse genetics, complementation analyses, and cell biological approaches, we assessed the in vivo function of two actin turnover proteins: actin interacting protein1 (AIP1) and actin depolymerizing factor (ADF). We report that AIP1 is a single-copy gene in the moss Physcomitrella patens. AIP1 knockout plants are viable but have reduced expansion of tip-growing cells. AIP1 is diffusely cytosolic and functions in a common genetic pathway with ADF to promote tip growth. Specifically, ADF can partially compensate for loss of AIP1, and AIP1 requires ADF for function. Consistent with a role in actin remodeling, AIP1 knockout lines accumulate F-actin bundles, have fewer dynamic ends, and have reduced severing frequency. Importantly, we demonstrate that AIP1 promotes and ADF is essential for cortical F-actin dynamics.
Figures





References
-
- Aizawa H., Katadae M., Maruya M., Sameshima M., Murakami-Murofushi K., Yahara I. (1999). Hyperosmotic stress-induced reorganization of actin bundles in Dictyostelium cells over-expressing cofilin. Genes Cells 4: 311–324 - PubMed
-
- Andrianantoandro E., Pollard T.D. (2006). Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol. Cell 24: 13–23 - PubMed
-
- Augustine R.C., Vidali L., Kleinman K.P., Bezanilla M. (2008). Actin depolymerizing factor is essential for viability in plants, and its phosphoregulation is important for tip growth. Plant J. 54: 863–875 - PubMed
-
- Balcer H.I., Goodman A.L., Rodal A.A., Smith E., Kugler J., Heuser J.E., Goode B.L. (2003). Coordinated regulation of actin filament turnover by a high-molecular-weight Srv2/CAP complex, cofilin, profilin, and Aip1. Curr. Biol. 13: 2159–2169 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

