Ectopic proliferation contributes to retinal dysplasia in the juvenile zebrafish patched2 mutant eye
- PMID: 22003118
- PMCID: PMC3231842
- DOI: 10.1167/iovs.11-8033
Ectopic proliferation contributes to retinal dysplasia in the juvenile zebrafish patched2 mutant eye
Abstract
Purpose: Patched is a well-studied tumor suppressor and negative regulator of the Hedgehog (Hh) pathway. Earlier work in this laboratory has shown that embryonic zebrafish patched2 (ptc2) mutant retinas possess an expanded ciliary marginal zone (CMZ) and phenotypes similar to those in human patients with basal cell naevus syndrome (BCNS), a congenital disorder linked to mutations in the human PTCH gene. This study extends the analysis of retinal structure and homeostasis in ptc2-/- mutants to juvenile stages, to determine whether Patched 2 function is essential in the postembryonic eye.
Methods: Histologic, immunohistochemical, and molecular analyses were used to characterize retinal defects in the 6-week-old juvenile ptc2-/- retina.
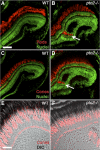
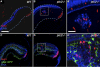
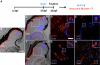
Results: Juvenile ptc2-/- mutants exhibited peripheral retinal dysplasias that included the presence of ectopic neuronal clusters in the inner nuclear layer (INL) and regions of disrupted retinal lamination. Retinal dysplasias were locally associated with ectopic proliferation. BrdU/EdU labeling and immunohistochemistry assays demonstrated that a population of ectopically proliferating cells gave rise to the ectopic neuronal clusters in the INL of ptc2-/- mutants and that this contributed to retinal dysplasia in the mutant eye.
Conclusions: These results demonstrate a direct link between overproliferation and retinal dysplasia in the ptc2-/- juvenile retina and establish ectopic proliferation as the likely cellular underpinning of retinal dysplasia in juvenile ptc2-/- mutants.
Figures




Similar articles
-
Expanded progenitor populations, vitreo-retinal abnormalities, and Müller glial reactivity in the zebrafish leprechaun/patched2 retina.BMC Dev Biol. 2009 Oct 19;9:52. doi: 10.1186/1471-213X-9-52. BMC Dev Biol. 2009. PMID: 19840373 Free PMC article.
-
A molecular phenotype atlas of the zebrafish retina.J Neurocytol. 2001 Jul;30(7):593-654. doi: 10.1023/a:1016516818393. J Neurocytol. 2001. PMID: 12118163
-
Retinal regional differences in photoreceptor cell death and regeneration in light-lesioned albino zebrafish.Exp Eye Res. 2006 Apr;82(4):558-75. doi: 10.1016/j.exer.2005.08.015. Epub 2005 Sep 30. Exp Eye Res. 2006. PMID: 16199033
-
Photo-regulation of rod precursor cell proliferation.Exp Eye Res. 2019 Jan;178:148-159. doi: 10.1016/j.exer.2018.09.015. Epub 2018 Sep 27. Exp Eye Res. 2019. PMID: 30267656
-
Rod progenitor cells in the mature zebrafish retina.Adv Exp Med Biol. 2008;613:361-8. doi: 10.1007/978-0-387-74904-4_42. Adv Exp Med Biol. 2008. PMID: 18188965 Free PMC article. Review.
Cited by
-
Sox4 regulates choroid fissure closure by limiting Hedgehog signaling during ocular morphogenesis.Dev Biol. 2015 Mar 1;399(1):139-153. doi: 10.1016/j.ydbio.2014.12.026. Epub 2014 Dec 31. Dev Biol. 2015. PMID: 25557621 Free PMC article.
-
Retinal Stem Cell 'Retirement Plans': Growth, Regulation and Species Adaptations in the Retinal Ciliary Marginal Zone.Int J Mol Sci. 2021 Jun 18;22(12):6528. doi: 10.3390/ijms22126528. Int J Mol Sci. 2021. PMID: 34207050 Free PMC article. Review.
-
Development of the Vertebrate Eye and Retina.Prog Mol Biol Transl Sci. 2015;134:397-414. doi: 10.1016/bs.pmbts.2015.06.006. Epub 2015 Jul 2. Prog Mol Biol Transl Sci. 2015. PMID: 26310167 Free PMC article. Review.
References
-
- Ekker SC, Ungar AR, Greenstein P, et al. Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Curr Biol. 1995;5:944–955 - PubMed
-
- Ericson J, Muhr J, Placzek M, Lints T, Jessell TM, Edlund T. Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell. 1995;81:747–756 - PubMed
-
- Dahmane N, Ruiz i Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–3100 - PubMed
-
- Ohnuma S, Hopper S, Wang KC, Philpott A, Harris WA. Co-ordinating retinal histogenesis: early cell cycle exit enhances early cell fate determination in the Xenopus retina. Development. 2002;129:2435–2446 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

