IL-23 is required for long-term control of Mycobacterium tuberculosis and B cell follicle formation in the infected lung
- PMID: 22003199
- PMCID: PMC3208087
- DOI: 10.4049/jimmunol.1101377
IL-23 is required for long-term control of Mycobacterium tuberculosis and B cell follicle formation in the infected lung
Abstract
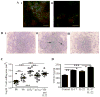
IL-23 is required for the IL-17 response to infection with Mycobacterium tuberculosis, but is not required for the early control of bacterial growth. However, mice deficient for the p19 component of IL-23 (Il23a(-/-)) exhibit increased bacterial growth late in infection that is temporally associated with smaller B cell follicles in the lungs. Cxcl13 is required for B cell follicle formation and immunity during tuberculosis. The absence of IL-23 results in decreased expression of Cxcl13 within M. tuberculosis-induced lymphocyte follicles in the lungs, and this deficiency was associated with increased cuffing of T cells around the vessels in the lungs of these mice. Il23a(-/-) mice also poorly expressed IL-17A and IL-22 mRNA. These cytokines were able to induce Cxcl13 in mouse primary lung fibroblasts, suggesting that these cytokines are likely involved in B cell follicle formation. Indeed, IL-17RA-deficient mice generated smaller B cell follicles early in the response, whereas IL-22-deficient mice had smaller B cell follicles at an intermediate time postinfection; however, only Il23a(-/-) mice had a sustained deficiency in B cell follicle formation and reduced immunity. We propose that in the absence of IL-23, expression of long-term immunity to tuberculosis is compromised due to reduced expression of Cxcl13 in B cell follicles and reduced ability of T cells to migrate from the vessels and into the lesion. Further, although IL-17 and IL-22 can both contribute to Cxcl13 production and B cell follicle formation, it is IL-23 that is critical in this regard.
Figures
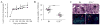



References
-
- Tsai M, Chakravarty S, Zhu G, Xu J, Tanaka K, Koch C, Tufariello J, Flynn J, Chan J. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell Microbiol. 2006;8:218–232. - PubMed
-
- Ulrichs T, Kosmiadi G, Trusov V, Jörg S, Pradl L, Titukhina M, Mishenko V, Gushina N, Kaufmann S. Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. J Pathol. 2004;204:217–228. - PubMed
-
- Maglione P, Xu J, Chan J. B cells moderate inflammatory progression and enhance bacterial containment upon pulmonary challenge with Mycobacterium tuberculosis. J Immunol. 2007;178:7222–7234. - PubMed
-
- Maglione P, Xu J, Casadevall A, Chan J. Fc{gamma} receptors regulate immune activation and susceptibility during Mycobacterium tuberculosis infection. J Immunol. 2008;180:3329–3338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AI046530/AI/NIAID NIH HHS/United States
- AI069121/AI/NIAID NIH HHS/United States
- R01 HL105427/HL/NHLBI NIH HHS/United States
- U54 AI057158/AI/NIAID NIH HHS/United States
- HL69409/HL/NHLBI NIH HHS/United States
- R01 AI069121/AI/NIAID NIH HHS/United States
- R01 HL069409/HL/NHLBI NIH HHS/United States
- AI083541/AI/NIAID NIH HHS/United States
- R37 HL079142/HL/NHLBI NIH HHS/United States
- R21 AI083541/AI/NIAID NIH HHS/United States
- HL-105427/HL/NHLBI NIH HHS/United States
- AI057158/AI/NIAID NIH HHS/United States
- AI46530/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

