MicroRNA-125b potentiates macrophage activation
- PMID: 22003200
- PMCID: PMC3208133
- DOI: 10.4049/jimmunol.1102001
MicroRNA-125b potentiates macrophage activation
Abstract
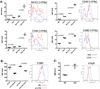
MicroRNA (miR)-125b expression is modulated in macrophages in response to stimulatory cues. In this study, we report a functional role of miR-125b in macrophages. We found that miR-125b is enriched in macrophages compared with lymphoid cells and whole immune tissues. Enforced expression of miR-125b drives macrophages to adapt an activated morphology that is accompanied by increased costimulatory factor expression and elevated responsiveness to IFN-γ, whereas anti-miR-125b treatment decreases CD80 surface expression. To determine whether these alterations in cell signaling, gene expression, and morphology have functional consequences, we examined the ability of macrophages with enhanced miR-125b expression to present Ags and found that they better stimulate T cell activation than control macrophages. Further indicating increased function, these macrophages were more effective at killing EL4 tumor cells in vitro and in vivo. Moreover, miR-125b repressed IFN regulatory factor 4 (IRF4), and IRF4 knockdown in macrophages mimicked the miR-125b overexpression phenotype. In summary, our evidence suggests that miR-125b is at least partly responsible for generating the activated nature of macrophages, at least partially by reducing IRF4 levels, and potentiates the functional role of macrophages in inducing immune responses.
Figures
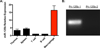



References
-
- Murphy KP, Travers P, Walport M, Janeway C. Janeway's immunobiology. New York: Garland Science; 2008.
-
- Kindt TJ, Goldsby RA, Osborne BA, Kuby J. Kuby immunology. New York: W.H. Freeman; 2007.
-
- O'Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010;10:111–122. - PubMed
-
- O'Neill LA, Sheedy FJ, McCoy CE. MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat Rev Immunol. 2011;11:163–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

