Synthesis and in vitro antiproliferative activity of novel (4-chloro- and 4-acyloxy-2-butynyl)thioquinolines
- PMID: 22003275
- PMCID: PMC3185227
- DOI: 10.1007/s00044-010-9495-y
Synthesis and in vitro antiproliferative activity of novel (4-chloro- and 4-acyloxy-2-butynyl)thioquinolines
Abstract
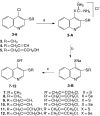
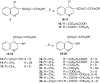
The series of new acetylenic thioquinolines containing propargyl, 4-chloro-2-butynyl, and 4-acyloxy-2-butynyl groups have been prepared and tested for antiproliferative activity in vitro against human [SW707 (colorectal adenocarcinoma), CCRF/CEM (leukemia), T47D (breast cancer)] and murine [P388 (leukemia), B16 (melanoma)] cancer lines. Most of the obtained compounds exhibited antiproliferative activity, especially compounds 8, 12, and 21 showed the ID(50) values ranging from 0.4 to 3.8 μg/ml comparable to that of cisplatin used as reference compounds.
Figures

References
-
- Abele R, Abele E, Rubina K, Dzenitis O, Arsenyan P, Shestakova I, Nesterova A, Domracheva I, Popelis J, Grinberga S, Lukevics E. Synthesis and cytotoxicity of 3-(hetarylothio)-1-propynyl-(trimethyl)silane. Chem Heterocycl Comp. 2002;38:867–872. doi: 10.1023/A:1020650224525. - DOI
-
- Bailey WJ, Fujiwara E. Mixed dihalides and halohydrins from butynediol. J Chem Soc. 1955;77:165–166. doi: 10.1021/ja01606a049. - DOI
-
- Ben-Zvi Z, Danon A. The chemistry of functional groups, supplement C2. In: Patai S, editor. The chemistry of triple bonded functional groups. New York: Wiley; 1994. pp. 739–788.
LinkOut - more resources
Full Text Sources
