Safety of indacaterol in the treatment of patients with COPD
- PMID: 22003293
- PMCID: PMC3186746
- DOI: 10.2147/COPD.S23816
Safety of indacaterol in the treatment of patients with COPD
Abstract
Purpose: Pooled data were analyzed to evaluate the safety and tolerability of indacaterol, a once-daily inhaled long-acting β(2)-agonist for chronic obstructive pulmonary disease (COPD).
Patients and methods: Data were pooled from clinical studies of 3-12 months' duration in patients with moderate-to-severe COPD receiving double-blind indacaterol 75 μg (n = 449), 150 μg (n = 2611), 300 μg (n = 1157), or 600 μg once daily (n = 547); formoterol 12 μg twice daily (n = 556); salmeterol 50 μg twice daily (n = 895); placebo (n = 2012); or tiotropium 18 μg once daily, given open label or blinded (n = 1214). Outcomes were adverse events, serious adverse events and deaths, plasma potassium, blood glucose, and QTc interval and vital signs.
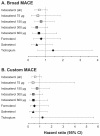
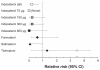
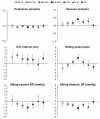
Results: The commonest adverse events with indacaterol were COPD worsening, nasopharyngitis, and headache; most cases were mild or moderate and incidence was generally similar to placebo and other active treatments. The risk of acute respiratory serious adverse events (leading to hospitalization, intubation, or death) was not significantly increased with any of the active treatments compared with placebo. COPD exacerbation rates (analyzed in the intent-to-treat population) were significantly reduced with all active treatments versus placebo. Hazard ratios versus placebo for major cardiovascular adverse events were < 1 for all indacaterol doses. Notable values for vital signs and measures of systemic β(2)-adrenoceptor activity were rare with indacaterol. The number of deaths adjusted per patient-year was lower with indacaterol (all doses combined) than with placebo (relative risk 0.21 [95% confidence interval 0.07-0.660], P = 0.008).
Conclusion: Indacaterol has a good profile of safety and tolerability that is appropriate for the maintenance treatment of patients with COPD.
Keywords: formoterol; indacaterol; safety; salmeterol; tiotropium; tolerability.
Figures

References
-
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2010. [Accessed March 3, 2011]. Available from: http://www.goldcopd.org.
-
- Dahl R, Chung KF, Buhl R, et al. INVOLVE (INdacaterol: Value in COPD: Longer Term Validation of Efficacy and Safety) Study Investigators. Efficacy of a new once-daily long-acting inhaled β2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax. 2010;65(6):473–479. - PubMed
-
- Donohue JF, Fogarty C, Lötvall J, et al. INHANCE Study Investigators. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182(2):155–162. - PubMed
-
- Kornmann O, Dahl R, Centanni S, et al. INLIGHT-2 (Indacaterol Efficacy Evaluation Using 150-μg Doses with COPD Patients) Study Investigators. Once-daily indacaterol versus twice-daily salmeterol for COPD: a placebo-controlled comparison. Eur Respir J. 2011;37(2):273–279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

