Adenovirus 5-vectored P. falciparum vaccine expressing CSP and AMA1. Part A: safety and immunogenicity in seronegative adults
- PMID: 22003383
- PMCID: PMC3189181
- DOI: 10.1371/journal.pone.0024586
Adenovirus 5-vectored P. falciparum vaccine expressing CSP and AMA1. Part A: safety and immunogenicity in seronegative adults
Abstract
Background: Models of immunity to malaria indicate the importance of CD8+ T cell responses for targeting intrahepatic stages and antibodies for targeting sporozoite and blood stages. We designed a multistage adenovirus 5 (Ad5)-vectored Plasmodium falciparum malaria vaccine, aiming to induce both types of responses in humans, that was tested for safety and immunogenicity in a Phase 1 dose escalation trial in Ad5-seronegative volunteers.
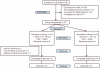
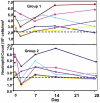
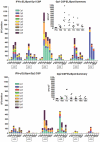
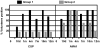
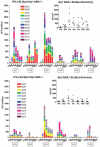
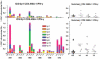
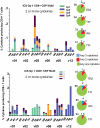
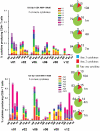
Methodology/principal findings: The NMRC-M3V-Ad-PfCA vaccine combines two adenovectors encoding circumsporozoite protein (CSP) and apical membrane antigen-1 (AMA1). Group 1 (n = 6) healthy volunteers received one intramuscular injection of 2×10∧10 particle units (1×10∧10 each construct) and Group 2 (n = 6) a five-fold higher dose. Transient, mild to moderate adverse events were more pronounced with the higher dose. ELISpot responses to CSP and AMA1 peaked at 1 month, were higher in the low dose (geomean CSP = 422, AMA1 = 862 spot forming cells/million) than in the high dose (CSP = 154, p = 0.049, AMA1 = 423, p = 0.045) group and were still positive at 12 months in a number of volunteers. ELISpot depletion assays identified dependence on CD4+ or on both CD4+ and CD8+ T cells, with few responses dependent only on CD8+ T cells. Intracellular cytokine staining detected stronger CD8+ than CD4+ T cell IFN-γ responses (CSP p = 0.0001, AMA1 p = 0.003), but similar frequencies of multifunctional CD4+ and CD8+ T cells secreting two or more of IFN-γ, TNF-α or IL-2. Median fluorescence intensities were 7-10 fold higher in triple than single secreting cells. Antibody responses were low but trended higher in the high dose group and did not inhibit growth of cultured P. falciparum blood stage parasites.
Significance: As found in other trials, adenovectored vaccines appeared safe and well-tolerated at doses up to 1×10∧11 particle units. This is the first demonstration in humans of a malaria vaccine eliciting strong CD8+ T cell IFN-γ responses.
Trial registration: ClinicalTrials.govNCT00392015.
Conflict of interest statement
Figures



References
-
- Clyde DF. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am J Trop Med Hyg. 1975;24:397–401. - PubMed
-
- Sigler CI, Leland P, Hollingdale MR. In vitro infectivity of irradiated Plasmodium berghei sporozoites to cultured hepatoma cells. Am J Trop Med Hyg. 1984;33:544–547. - PubMed
-
- Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967;216:160–162. - PubMed
-
- Doolan DL, Martinez-Alier N. Immune response to pre-erythrocytic stages of malaria parasites. Curr Mol Med. 2006;6:169–185. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

