Adenovirus-5-vectored P. falciparum vaccine expressing CSP and AMA1. Part B: safety, immunogenicity and protective efficacy of the CSP component
- PMID: 22003411
- PMCID: PMC3189219
- DOI: 10.1371/journal.pone.0025868
Adenovirus-5-vectored P. falciparum vaccine expressing CSP and AMA1. Part B: safety, immunogenicity and protective efficacy of the CSP component
Abstract
Background: A protective malaria vaccine will likely need to elicit both cell-mediated and antibody responses. As adenovirus vaccine vectors induce both these responses in humans, a Phase 1/2a clinical trial was conducted to evaluate the efficacy of an adenovirus serotype 5-vectored malaria vaccine against sporozoite challenge.
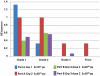
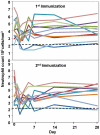
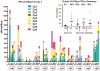
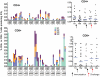
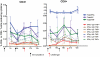
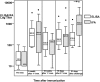
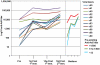
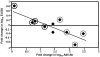
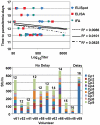
Methodology/principal findings: NMRC-MV-Ad-PfC is an adenovirus vector encoding the Plasmodium falciparum 3D7 circumsporozoite protein (CSP). It is one component of a two-component vaccine NMRC-M3V-Ad-PfCA consisting of one adenovector encoding CSP and one encoding apical membrane antigen-1 (AMA1) that was evaluated for safety and immunogenicity in an earlier study (see companion paper, Sedegah et al). Fourteen Ad5 seropositive or negative adults received two doses of NMRC-MV-Ad-PfC sixteen weeks apart, at 1 x 1010 particle units per dose. The vaccine was safe and well tolerated. All volunteers developed positive ELISpot responses by 28 days after the first immunization (geometric mean 272 spot forming cells/million[sfc/m]) that declined during the following 16 weeks and increased after the second dose to levels that in most cases were less than the initial peak (geometric mean 119 sfc/m). CD8+ predominated over CD4+ responses, as in the first clinical trial. Antibody responses were poor and like ELISpot responses increased after the second immunization but did not exceed the initial peak. Pre-existing neutralizing antibodies (NAb) to Ad5 did not affect the immunogenicity of the first dose, but the fold increase in NAb induced by the first dose was significantly associated with poorer antibody responses after the second dose, while ELISpot responses remained unaffected. When challenged by the bite of P. falciparum-infected mosquitoes, two of 11 volunteers showed a delay in the time to patency compared to infectivity controls, but no volunteers were sterilely protected.
Significance: The NMRC-MV-Ad-PfC vaccine expressing CSP was safe and well tolerated given as two doses, but did not provide sterile protection.
Trial registration: ClinicalTrials.gov NCT00392015.
Conflict of interest statement
Figures
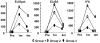

References
-
- WHO World Malaria Report. 2009. http://wwwwhoint/malaria/world_malaria_report_2009/en/indexhtml.
-
- Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, et al. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. - PubMed
-
- Sigler CI, Leland P, Hollingdale MR. In vitro infectivity of irradiated Plasmodium berghei sporozoites to cultured hepatoma cells. Am J Trop Med Hyg. 1984;33:544–547. - PubMed
-
- Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967;216:160–162. - PubMed
-
- Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–1164. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

