Binding-folding induced regulation of AF1 transactivation domain of the glucocorticoid receptor by a cofactor that binds to its DNA binding domain
- PMID: 22003412
- PMCID: PMC3189220
- DOI: 10.1371/journal.pone.0025875
Binding-folding induced regulation of AF1 transactivation domain of the glucocorticoid receptor by a cofactor that binds to its DNA binding domain
Abstract
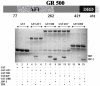
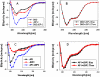
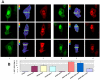
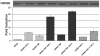
Intrinsically disordered (ID) regions of proteins commonly exist within transcription factors, including the N-terminal domain (NTD) of steroid hormone receptors (SHRs) that possesses a powerful activation function, AF1 region. The mechanisms by which SHRs pass signals from a steroid hormone to control gene expression remain a central unresolved problem. The role of N-terminal activation function AF1, which exists in an intrinsically disordered (ID) conformation, in this process is of immense importance. It is hypothesized that under physiological conditions, ID AF1 undergoes disorder/order transition via inter- and intra-molecular communications, which allows AF1 surfaces to interact with specific co-regulatory proteins, critical for the final outcome of target gene expression regulated by SHRs. However, the means by which AF1 acquires functionally folded conformations is not well understood. In this study, we tested whether binding of jun dimerization protein 2 (JDP2) within the DNA binding domain (DBD) of the glucocorticoid receptor (GR) leads to acquisition of functionally active structure in its AF1/NTD. Our results show that signals mediated from GR DBD:JDP2 interactions in a two domain GR fragment, consisting of the entire NTD and little beyond DBD, significantly increased secondary/tertiary structure formation in the NTD/AF1. This increased structure formation facilitated AF1's interaction with specific co-regulatory proteins and subsequent glucocorticoid response element-mediated AF1 promoter:reporter activity. These results support the hypothesis that inter- and intra-molecular signals give a functionally active structure(s) to the GR AF1, which is important for its transcriptional activity.
Conflict of interest statement
Figures

Similar articles
-
TBP binding-induced folding of the glucocorticoid receptor AF1 domain facilitates its interaction with steroid receptor coactivator-1.PLoS One. 2011;6(7):e21939. doi: 10.1371/journal.pone.0021939. Epub 2011 Jul 7. PLoS One. 2011. PMID: 21760925 Free PMC article.
-
Site-specific phosphorylation induces functionally active conformation in the intrinsically disordered N-terminal activation function (AF1) domain of the glucocorticoid receptor.Mol Cell Biol. 2010 Jan;30(1):220-30. doi: 10.1128/MCB.00552-09. Mol Cell Biol. 2010. PMID: 19841061 Free PMC article.
-
Binding of the N-terminal region of coactivator TIF2 to the intrinsically disordered AF1 domain of the glucocorticoid receptor is accompanied by conformational reorganizations.J Biol Chem. 2012 Dec 28;287(53):44546-60. doi: 10.1074/jbc.M112.411330. Epub 2012 Nov 6. J Biol Chem. 2012. PMID: 23132854 Free PMC article.
-
Role of Phosphorylation in the Modulation of the Glucocorticoid Receptor's Intrinsically Disordered Domain.Biomolecules. 2019 Mar 11;9(3):95. doi: 10.3390/biom9030095. Biomolecules. 2019. PMID: 30862072 Free PMC article. Review.
-
Folding of the glucocorticoid receptor N-terminal transactivation function: dynamics and regulation.Mol Cell Endocrinol. 2012 Jan 30;348(2):450-6. doi: 10.1016/j.mce.2011.03.024. Epub 2011 Apr 8. Mol Cell Endocrinol. 2012. PMID: 21501657 Review.
Cited by
-
Amyloid Fibrils of Pisum sativum L. Vicilin Inhibit Pathological Aggregation of Mammalian Proteins.Int J Mol Sci. 2023 Aug 18;24(16):12932. doi: 10.3390/ijms241612932. Int J Mol Sci. 2023. PMID: 37629113 Free PMC article.
-
Phosphorylation-Regulated Conformational Diversity and Topological Dynamics of an Intrinsically Disordered Nuclear Receptor.J Phys Chem B. 2025 Jul 31;129(30):7719-7730. doi: 10.1021/acs.jpcb.5c03257. Epub 2025 Jul 17. J Phys Chem B. 2025. PMID: 40673718 Free PMC article.
-
Regulation of the structurally dynamic N-terminal domain of progesterone receptor by protein-induced folding.J Biol Chem. 2013 Oct 18;288(42):30285-30299. doi: 10.1074/jbc.M113.491787. Epub 2013 Aug 30. J Biol Chem. 2013. PMID: 23995840 Free PMC article.
-
Agonism/antagonism switching in allosteric ensembles.Proc Natl Acad Sci U S A. 2012 Mar 13;109(11):4134-9. doi: 10.1073/pnas.1120519109. Epub 2012 Mar 2. Proc Natl Acad Sci U S A. 2012. PMID: 22388747 Free PMC article.
-
Identification of mineralocorticoid receptor target genes in the mouse hippocampus.J Neuroendocrinol. 2019 Aug;31(8):e12735. doi: 10.1111/jne.12735. Epub 2019 Jun 14. J Neuroendocrinol. 2019. PMID: 31121060 Free PMC article.
References
-
- Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335–344. - PubMed
-
- Yamamoto KR. Steroid receptor regulated transcription of specific genes and gene network. Ann Rev Genet. 1985;19:209–252. - PubMed
-
- Kumar R, Thompson EB. Gene regulation by the glucocorticoid receptor: structure:function relationship. J Steroid Biochem Mol Biol. 2005;94:383–394. - PubMed
-
- Kumar R, Thompson EB. The structure of the nuclear hormone receptors. Steroids. 1999;64:310–319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

