Lipopolysaccharide enhances transforming growth factor β1-induced platelet-derived growth factor-B expression in bile duct epithelial cells
- PMID: 22004089
- PMCID: PMC3262076
- DOI: 10.1111/j.1440-1746.2011.06941.x
Lipopolysaccharide enhances transforming growth factor β1-induced platelet-derived growth factor-B expression in bile duct epithelial cells
Abstract
Background and aim: Platelet-derived growth factor (PDGF)-B is a potent profibrogenic mediator expressed by bile duct epithelial cells (BDECs) that contributes to liver fibrosis after bile duct ligation. However, the mechanism of PDGF-B induction in BDECs during cholestasis is not known. Transforming growth factor β (TGFβ) and lipopolysaccharide (LPS) also contribute to the profibrogenic response after bile duct ligation. We tested the hypothesis that LPS and TGFβ1 synergistically induce PDGF-B expression in BDECs.
Methods: Transformed human BDECs (MMNK-1 cells) and primary rat BDECs were stimulated with LPS and/or TGFβ1, and signaling pathways through which LPS potentiates TGFβ1-induced PDGF-B mRNA expression were investigated.
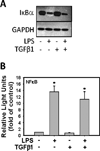
Results: Stimulation of MMNK-1 cells with LPS alone did not significantly induce PDGF-B mRNA expression. However, LPS co-treatment enhanced TGFβ1 induction of PDGF-B mRNA in MMNK-1 cells and also in primary rat BDECs. Importantly, co-treatment of MMNK-1 cells with LPS and TGFβ1 also significantly increased PDGF-BB protein expression. Interestingly, LPS did not affect TGFβ1 activation of a SMAD-dependent reporter construct. Rather, stimulation of MMNK-1 cells with LPS, but not TGFβ1, increased JNK1/2 phosphorylation. Expression of dominant negative JNK2, but not dominant negative JNK1, inhibited the LPS potentiation of TGFβ1-induced PDGF-B mRNA expression in MMNK-1 cells. In addition, LPS treatment caused IκBα degradation and activation of a nuclear factor κB (NFκB)-dependent reporter construct. Expression of an IκBα super repressor inhibited activation of NFκB and attenuated LPS potentiation of TGFβ1-induced PDGF-B mRNA.
Conclusions: The results indicate that LPS activation of NFκB and JNK2 enhances TGFβ1-induced PDGF-B expression in BDECs.
© 2011 Journal of Gastroenterology and Hepatology Foundation and Blackwell Publishing Asia Pty Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures




Similar articles
-
Regulation of transforming growth factor-β1-dependent integrin β6 expression by p38 mitogen-activated protein kinase in bile duct epithelial cells.J Pharmacol Exp Ther. 2011 May;337(2):471-8. doi: 10.1124/jpet.110.177337. Epub 2011 Feb 8. J Pharmacol Exp Ther. 2011. PMID: 21303922 Free PMC article.
-
Effects of lipopolysaccharide on platelet-derived growth factor isoform and receptor expression in cultured rat common bile duct fibroblasts and cholangiocytes.J Gastroenterol Hepatol. 2009 Jul;24(7):1218-25. doi: 10.1111/j.1440-1746.2008.05729.x. J Gastroenterol Hepatol. 2009. PMID: 19691150
-
Statin pretreatment inhibits the lipopolysaccharide-induced epithelial-mesenchymal transition via the downregulation of toll-like receptor 4 and nuclear factor-κB in human biliary epithelial cells.J Gastroenterol Hepatol. 2016 Jun;31(6):1220-8. doi: 10.1111/jgh.13230. J Gastroenterol Hepatol. 2016. PMID: 26574150
-
Platelet-Derived Growth Factor and Transforming Growth Factor β1 Regulate ARDS-Associated Lung Fibrosis Through Distinct Signaling Pathways.Cell Physiol Biochem. 2015;36(3):937-46. doi: 10.1159/000430268. Epub 2015 Jun 12. Cell Physiol Biochem. 2015. PMID: 26088859
-
Docosahexaenoic acid attenuates LPS-stimulated inflammatory response by regulating the PPARγ/NF-κB pathways in primary bovine mammary epithelial cells.Res Vet Sci. 2017 Jun;112:7-12. doi: 10.1016/j.rvsc.2016.12.011. Epub 2017 Jan 5. Res Vet Sci. 2017. PMID: 28095338 Review.
Cited by
-
Longitudinal in vivo bioimaging of hepatocyte transcription factor activity following cholestatic liver injury in mice.Sci Rep. 2017 Feb 3;7:41874. doi: 10.1038/srep41874. Sci Rep. 2017. PMID: 28157201 Free PMC article.
-
JNK2 regulates vascular remodeling in pulmonary hypertension.Pulm Circ. 2018 Jul-Sep;8(3):2045894018778156. doi: 10.1177/2045894018778156. Epub 2018 May 2. Pulm Circ. 2018. PMID: 29718758 Free PMC article.
References
-
- Pierce GF, Mustoe TA, Altrock BW, Deuel TF, Thomason A. Role of platelet-derived growth factor in wound healing. J. Cell Biochem. 1991;45:319–326. - PubMed
-
- Czochra P, Klopcic B, Meyer E, et al. Liver fibrosis induced by hepatic overexpression of PDGF-B in transgenic mice. J. Hepatol. 2006;45:419–428. - PubMed
-
- Isbrucker RA, Peterson TC. Platelet-derived growth factor and pentoxifylline modulation of collagen synthesis in myofibroblasts. Toxicol. Appl. Pharmacol. 1998;149:120–126. - PubMed
-
- Kinnman N, Francoz C, Barbu V, et al. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Invest. 2003;83:163–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

