CSPG4, a potential therapeutic target, facilitates malignant progression of melanoma
- PMID: 22004131
- PMCID: PMC3426219
- DOI: 10.1111/j.1755-148X.2011.00929.x
CSPG4, a potential therapeutic target, facilitates malignant progression of melanoma
Abstract
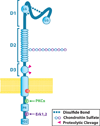
Chondroitin sulfate proteoglycan 4 (CSPG4), a transmembrane proteoglycan originally identified as a highly immunogenic tumor antigen on the surface of melanoma cells, is associated with melanoma tumor formation and poor prognosis in certain melanomas and several other tumor types. The complex mechanisms by which CSPG4 affects melanoma progression have started to be defined, in particular the association with other cell surface proteins and receptor tyrosine kinases (RTKs) and its central role in modulating the function of these proteins. CSPG4 is essential to the growth of melanoma tumors through its modulation of integrin function and enhanced growth factor receptor-regulated pathways including sustained activation of ERK 1,2. This activation of integrin, RTK, and ERK1,2 function by CSPG4 modulates numerous aspects of tumor progression. CSPG4 expression has further been correlated to resistance of melanoma to conventional chemotherapeutics. This review outlines recent advances in our understanding of CSPG4-associated cell signaling, describing the central role it plays in melanoma tumor cell growth, motility, and survival, and explores how modifying CSPG4 function and protein-protein interactions may provide us with novel combinatorial therapies for the treatment of advanced melanoma.
2011 John Wiley & Sons A/S.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

