Positive feedback loops for factor V and factor VII activation supply sensitivity to local surface tissue factor density during blood coagulation
- PMID: 22004734
- PMCID: PMC3192965
- DOI: 10.1016/j.bpj.2011.08.034
Positive feedback loops for factor V and factor VII activation supply sensitivity to local surface tissue factor density during blood coagulation
Abstract
Blood coagulation is triggered not only by surface tissue factor (TF) density but also by surface TF distribution. We investigated recognition of surface TF distribution patterns during blood coagulation and identified the underlying molecular mechanisms. For these investigations, we employed 1), an in vitro reaction-diffusion experimental model of coagulation; and 2), numerical simulations using a mathematical model of coagulation in a three-dimensional space. When TF was uniformly immobilized over the activating surface, the clotting initiation time in normal plasma increased from 4 min to >120 min, with a decrease in TF density from 100 to 0.7 pmol/m(2). In contrast, surface-immobilized fibroblasts initiated clotting within 3-7 min, independently of fibroblast quantity and despite a change in average surface TF density from 0.5 to 130 pmol/m(2). Experiments using factor V-, VII-, and VIII-deficient plasma and computer simulations demonstrated that different responses to these two TF distributions are caused by two positive feedback loops in the blood coagulation network: activation of the TF-VII complex by factor Xa, and activation of factor V by thrombin. This finding suggests a new role for these reactions: to supply sensitivity to local TF density during blood coagulation.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


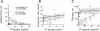




Similar articles
-
Blood flow controls coagulation onset via the positive feedback of factor VII activation by factor Xa.BMC Syst Biol. 2010 Jan 26;4:5. doi: 10.1186/1752-0509-4-5. BMC Syst Biol. 2010. PMID: 20102623 Free PMC article.
-
Activated factor X and thrombin formation triggered by tissue factor on endothelial cell matrix in a flow model: effect of the tissue factor pathway inhibitor.Blood. 1994 Aug 15;84(4):1132-42. Blood. 1994. PMID: 8049429
-
Initiation of the extrinsic pathway of coagulation by human and rabbit alveolar macrophages: a kinetic study.Blood. 1989 Oct;74(5):1583-90. Blood. 1989. PMID: 2790188
-
Formation of the fibrin clot: the balance of procoagulant and inhibitory factors.Clin Haematol. 1985 Jun;14(2):281-342. Clin Haematol. 1985. PMID: 2994929 Review.
-
The lipoprotein-associated coagulation inhibitor.Prog Hemost Thromb. 1991;10:243-68. Prog Hemost Thromb. 1991. PMID: 2008533 Review.
Cited by
-
Systems Biology Approach for Personalized Hemostasis Correction.J Pers Med. 2022 Nov 15;12(11):1903. doi: 10.3390/jpm12111903. J Pers Med. 2022. PMID: 36422079 Free PMC article.
-
Sensitivity and Robustness of Spatially Dependent Thrombin Generation and Fibrin Clot Propagation.Biophys J. 2018 Dec 18;115(12):2461-2473. doi: 10.1016/j.bpj.2018.11.009. Epub 2018 Nov 14. Biophys J. 2018. PMID: 30514632 Free PMC article.
-
Reversing direct factor Xa or thrombin inhibitors: Factor V addition to prothrombin complex concentrate is beneficial in vitro.Res Pract Thromb Haemost. 2022 Apr 25;6(3):e12699. doi: 10.1002/rth2.12699. eCollection 2022 Mar. Res Pract Thromb Haemost. 2022. PMID: 35494506 Free PMC article.
-
Thrombodynamics-A new global hemostasis assay for heparin monitoring in patients under the anticoagulant treatment.PLoS One. 2018 Jun 28;13(6):e0199900. doi: 10.1371/journal.pone.0199900. eCollection 2018. PLoS One. 2018. PMID: 29953528 Free PMC article.
-
Co-ordinated spatial propagation of blood plasma clotting and fibrinolytic fronts.PLoS One. 2017 Jul 7;12(7):e0180668. doi: 10.1371/journal.pone.0180668. eCollection 2017. PLoS One. 2017. PMID: 28686711 Free PMC article.
References
-
- Monroe D.M., Key N.S. The tissue factor-factor VIIa complex: procoagulant activity, regulation, and multitasking. J. Thromb. Haemost. 2007;5:1097–1105. - PubMed
-
- Butenas S., Mann K.G. Kinetics of human factor VII activation. Biochemistry. 1996;35:1904–1910. - PubMed
-
- Hemker H.C., Al Dieri R., Béguin S. Thrombin generation, a function test of the haemostatic-thrombotic system. Thromb. Haemost. 2006;96:553–561. - PubMed
-
- Rand M.D., Lock J.B., Mann K.G. Blood clotting in minimally altered whole blood. Blood. 1996;88:3432–3445. - PubMed
-
- Monroe D.M., Hoffman M., Roberts H.R. Transmission of a procoagulant signal from tissue factor-bearing cell to platelets. Blood Coagul. Fibrinolysis. 1996;7:459–464. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

