Isoflurane alters the structure and dynamics of GLIC
- PMID: 22004744
- PMCID: PMC3192980
- DOI: 10.1016/j.bpj.2011.09.026
Isoflurane alters the structure and dynamics of GLIC
Abstract
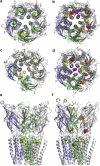
Pentameric ligand-gated ion channels are targets of general anesthetics. Although the search for discrete anesthetic binding sites has achieved some degree of success, little is known regarding how anesthetics work after the events of binding. Using the crystal structures of the bacterial Gloeobacter violaceus pentameric ligand-gated ion channel (GLIC), which is sensitive to a variety of general anesthetics, we performed multiple molecular dynamics simulations in the presence and absence of the general anesthetic isoflurane. Isoflurane bound to several locations within GLIC, including the transmembrane pocket identified crystallographically, the extracellular (EC) domain, and the interface of the EC and transmembrane domains. Isoflurane also entered the channel after the pore was dehydrated in one of the simulations. Isoflurane disrupted the quaternary structure of GLIC, as evidenced in a striking association between the binding and breakage of intersubunit salt bridges in the EC domain. The pore-lining helix experienced lateral and inward radial tilting motion that contributed to the channel closure. Isoflurane binding introduced strong anticorrelated motions between different subunits of GLIC. The demonstrated structural and dynamical modulations by isoflurane aid in the understanding of the underlying mechanism of anesthetic inhibition of GLIC and possibly other homologous pentameric ligand-gated ion channels.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Anesthetic binding in a pentameric ligand-gated ion channel: GLIC.Biophys J. 2010 Sep 22;99(6):1801-9. doi: 10.1016/j.bpj.2010.07.023. Biophys J. 2010. PMID: 20858424 Free PMC article.
-
Propofol binding to the resting state of the gloeobacter violaceus ligand-gated ion channel (GLIC) induces structural changes in the inter- and intrasubunit transmembrane domain (TMD) cavities.J Biol Chem. 2013 Jun 14;288(24):17420-31. doi: 10.1074/jbc.M113.464040. Epub 2013 May 2. J Biol Chem. 2013. PMID: 23640880 Free PMC article.
-
Common Internal Allosteric Network Links Anesthetic Binding Sites in a Pentameric Ligand-Gated Ion Channel.PLoS One. 2016 Jul 12;11(7):e0158795. doi: 10.1371/journal.pone.0158795. eCollection 2016. PLoS One. 2016. PMID: 27403526 Free PMC article.
-
Atomic structure and dynamics of pentameric ligand-gated ion channels: new insight from bacterial homologues.J Physiol. 2010 Feb 15;588(Pt 4):565-72. doi: 10.1113/jphysiol.2009.183160. Epub 2009 Dec 7. J Physiol. 2010. PMID: 19995852 Free PMC article. Review.
-
A gating mechanism of pentameric ligand-gated ion channels.Proc Natl Acad Sci U S A. 2013 Oct 15;110(42):E3987-96. doi: 10.1073/pnas.1313785110. Epub 2013 Sep 16. Proc Natl Acad Sci U S A. 2013. PMID: 24043807 Free PMC article. Review.
Cited by
-
Multiple functional neurosteroid binding sites on GABAA receptors.PLoS Biol. 2019 Mar 7;17(3):e3000157. doi: 10.1371/journal.pbio.3000157. eCollection 2019 Mar. PLoS Biol. 2019. PMID: 30845142 Free PMC article.
-
Pentameric Ligand-gated Ion Channels : Insights from Computation.Mol Simul. 2014 Apr 17;40(10-11):821-829. doi: 10.1080/08927022.2014.896462. Mol Simul. 2014. PMID: 25931676 Free PMC article.
-
The cellular membrane as a mediator for small molecule interaction with membrane proteins.Biochim Biophys Acta. 2016 Oct;1858(10):2290-2304. doi: 10.1016/j.bbamem.2016.04.016. Epub 2016 May 6. Biochim Biophys Acta. 2016. PMID: 27163493 Free PMC article.
-
Novel activation of voltage-gated K(+) channels by sevoflurane.J Biol Chem. 2012 Nov 23;287(48):40425-32. doi: 10.1074/jbc.M112.405787. Epub 2012 Oct 4. J Biol Chem. 2012. PMID: 23038249 Free PMC article.
-
Markov state models of proton- and pore-dependent activation in a pentameric ligand-gated ion channel.Elife. 2021 Oct 15;10:e68369. doi: 10.7554/eLife.68369. Elife. 2021. PMID: 34652272 Free PMC article.
References
-
- Campagna J.A., Miller K.W., Forman S.A. Mechanisms of actions of inhaled anesthetics. N. Engl. J. Med. 2003;348:2110–2124. - PubMed
-
- Hemmings H.C., Jr., Akabas M.H., Harrison N.L. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol. Sci. 2005;26:503–510. - PubMed
-
- Chiara D.C., Dangott L.J., Cohen J.B. Identification of nicotinic acetylcholine receptor amino acids photolabeled by the volatile anesthetic halothane. Biochemistry. 2003;42:13457–13467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

