Tumour macrophages as potential targets of bisphosphonates
- PMID: 22005011
- PMCID: PMC3215187
- DOI: 10.1186/1479-5876-9-177
Tumour macrophages as potential targets of bisphosphonates
Abstract
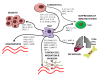
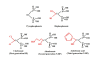
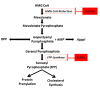
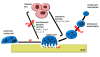
Tumour cells communicate with the cells of their microenvironment via a series of molecular and cellular interactions to aid their progression to a malignant state and ultimately their metastatic spread. Of the cells in the microenvironment with a key role in cancer development, tumour associated macrophages (TAMs) are among the most notable. Tumour cells release a range of chemokines, cytokines and growth factors to attract macrophages, and these in turn release numerous factors (e.g. VEGF, MMP-9 and EGF) that are implicated in invasion-promoting processes such as tumour cell growth, flicking of the angiogenic switch and immunosuppression. TAM density has been shown to correlate with poor prognosis in breast cancer, suggesting that these cells may represent a potential therapeutic target. However, there are currently no agents that specifically target TAM's available for clinical use.Bisphosphonates (BPs), such as zoledronic acid, are anti-resorptive agents approved for treatment of skeletal complication associated with metastatic breast cancer and prostate cancer. These agents act on osteoclasts, key cells in the bone microenvironment, to inhibit bone resorption. Over the past 30 years this has led to a great reduction in skeletal-related events (SRE's) in patients with advanced cancer and improved the morbidity associated with cancer-induced bone disease. However, there is now a growing body of evidence, both from in vitro and in vivo models, showing that zoledronic acid can also target tumour cells to increase apoptotic cell death and decrease proliferation, migration and invasion, and that this effect is significantly enhanced in combination with chemotherapy agents. Whether macrophages in the peripheral tumour microenvironment are exposed to sufficient levels of bisphosphonate to be affected is currently unknown. Macrophages belong to the same cell lineage as osteoclasts, the major target of BPs, and are highly phagocytic cells shown to be sensitive to bisphosphonates in model studies; In vitro, zoledronic acid causes increased apoptotic cell death; in vivo the drug has been shown to inhibit the production of pro-angiogenic factor MMP-9, as well as most recent evidence showing it can trigger the reversal of the TAMs phenotype from pro-tumoral M2 to tumoricidal M1. There is thus accumulating evidence supporting the hypothesis that effects on TAMs may contribute to the anti-tumour effect of bisphosphonates. This review will focus in detail on the role of tumour associated macrophages in breast cancer progression, the actions of bisphosphonates on macrophages in vitro and in tumour models in vivo and summarise the evidence supporting the potential for the targeting of tumour macrophages with bisphosphonates.
Figures

Similar articles
-
Anti-tumour activity of bisphosphonates in preclinical models of breast cancer.Breast Cancer Res. 2010;12(6):214. doi: 10.1186/bcr2769. Epub 2010 Dec 16. Breast Cancer Res. 2010. PMID: 21176176 Free PMC article. Review.
-
Macrophages as potential targets for zoledronic acid outside the skeleton-evidence from in vitro and in vivo models.Cell Oncol (Dordr). 2013 Dec;36(6):505-14. doi: 10.1007/s13402-013-0156-2. Epub 2013 Nov 1. Cell Oncol (Dordr). 2013. PMID: 24177992
-
The role of bisphosphonates in breast and prostate cancers.Endocr Relat Cancer. 2004 Jun;11(2):207-24. doi: 10.1677/erc.0.0110207. Endocr Relat Cancer. 2004. PMID: 15163299 Review.
-
Antitumor effects of bisphosphonates.Cancer. 2003 Feb 1;97(3 Suppl):840-7. doi: 10.1002/cncr.11128. Cancer. 2003. PMID: 12548584 Review.
-
Zoledronic acid impairs stromal reactivity by inhibiting M2-macrophages polarization and prostate cancer-associated fibroblasts.Oncotarget. 2017 Jan 3;8(1):118-132. doi: 10.18632/oncotarget.9497. Oncotarget. 2017. PMID: 27223431 Free PMC article.
Cited by
-
Research progress on the role of tumor‑associated macrophages in tumor development and their use as molecular targets (Review).Int J Oncol. 2024 Feb;64(2):11. doi: 10.3892/ijo.2023.5599. Epub 2023 Dec 8. Int J Oncol. 2024. PMID: 38063203 Free PMC article. Review.
-
Tumor-Associated Macrophages-Implications for Molecular Oncology and Imaging.Biomedicines. 2021 Apr 2;9(4):374. doi: 10.3390/biomedicines9040374. Biomedicines. 2021. PMID: 33918295 Free PMC article. Review.
-
Toward integrative cancer immunotherapy: targeting the tumor microenvironment.J Transl Med. 2012 Apr 10;10:70. doi: 10.1186/1479-5876-10-70. J Transl Med. 2012. PMID: 22490302 Free PMC article.
-
Effects of Epigallocatechin-3-Gallate on Matrix Metalloproteinases in Terms of Its Anticancer Activity.Molecules. 2023 Jan 5;28(2):525. doi: 10.3390/molecules28020525. Molecules. 2023. PMID: 36677584 Free PMC article. Review.
-
Nanotherapeutics to Modulate the Compromised Micro-Environment for Lung Cancers and Chronic Obstructive Pulmonary Disease.Front Pharmacol. 2018 Jul 16;9:759. doi: 10.3389/fphar.2018.00759. eCollection 2018. Front Pharmacol. 2018. PMID: 30061830 Free PMC article. Review.
References
-
- Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, Nur U, Tracey E, Coory M, Hatcher J, McGahan CE, Turner D, Marrett L, Gjerstorff ML, Johannesen TB, Adolfsson J, Lambe M, Lawrence G, Meechan D, Morris EJ, Middleton R, Steward J, Richards MA. ICBP Module 1 Working Group. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995-2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377(9760):127–38. doi: 10.1016/S0140-6736(10)62231-3. - DOI - PMC - PubMed
-
- Coffelt SB, Hughes R, Lewis CE. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochim Biophys Acta. 2009;1796(1):11–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

