Glutathione-S-transferase enhances proliferation-migration and protects against shikonin-induced cell death in breast cancer cells
- PMID: 22005156
- PMCID: PMC11916664
- DOI: 10.1016/j.kjms.2011.06.010
Glutathione-S-transferase enhances proliferation-migration and protects against shikonin-induced cell death in breast cancer cells
Abstract
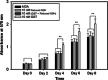
Glutathione-S-transferase (GST) is a cytoplasmic protein responsible for detoxification, but the effect of the enzyme on cell biological events, including proliferation and migration, has never been reported. Thus, we evaluated the detoxification effect of in vitro-applied GST on cancer cell proliferation and migration. Assays for proliferation and migration of human breast cancer cells in the presence of GST were carried out. Binding of GST on the surface of the cancer cells was studied by flow cytometry. Detoxification through GST pathway was studied in the presence of shikonin. The effective dosage of GST in enhancement of cell proliferation was 10-50 nM, and the cell migration could be significantly enhanced after 6 hours in the presence of 2-50 nM GST. Therefore, overall cell proliferation and migration could be enhanced in the presence of 10nM or greater concentration of GST, and 15 μM shikonin-induced toxification of the cancer cells could be neutralized by 1.0 μM GST. Flow cytometry showed that GST directly bound to the surface of the cancer cells, and this was confirmed by fluorescence confocal microscopic observation. It is concluded that human class π-GST enhances proliferation and migration of human breast cancer cells by means of direct binding to the cell surface and maintaining cell viability by detoxification.
Copyright © 2011. Published by Elsevier B.V.
Figures







References
-
- Mannervik B., Danielson U.H.. Glutathione transferases: structure and catalytic activity. CRC Crit Rev Biochem. 1988; 23: 283–337. - PubMed
-
- Andujar‐Sanchez M., Smith A.W., Clemente‐Jimenez J.M., Rodriguez‐Vico F., Heras‐Vazquez F.J.L., VJara‐Pérez V., et al. Crystallographic and thermodynamic analysis of the binding of S‐octylglutathione to the Tyr 7 to Phe mutant of glutathione S‐transferase from Schistosoma japonicum . Biochemistry. 2005; 44: 1174–1183. - PubMed
-
- Armstrong R.N.. Structure, catalytic mechanism, and evolution of the glutathione transferases. Chem Res Toxicol. 1997; 10: 2–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

