Calpain mediates pulmonary vascular remodeling in rodent models of pulmonary hypertension, and its inhibition attenuates pathologic features of disease
- PMID: 22005303
- PMCID: PMC3204840
- DOI: 10.1172/JCI57734
Calpain mediates pulmonary vascular remodeling in rodent models of pulmonary hypertension, and its inhibition attenuates pathologic features of disease
Abstract
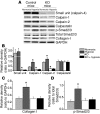
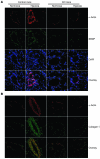
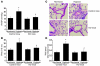
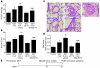
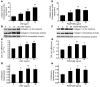
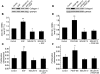
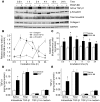
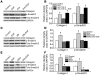
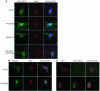
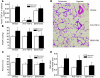
Pulmonary hypertension is a severe and progressive disease, a key feature of which is pulmonary vascular remodeling. Several growth factors, including EGF, PDGF, and TGF-β1, are involved in pulmonary vascular remodeling during pulmonary hypertension. However, increased knowledge of the downstream signaling cascades is needed if effective clinical interventions are to be developed. In this context, calpain provides an interesting candidate therapeutic target, since it is activated by EGF and PDGF and has been reported to activate TGF-β1. Thus, in this study, we examined the role of calpain in pulmonary vascular remodeling in two rodent models of pulmonary hypertension. These data showed that attenuated calpain activity in calpain-knockout mice or rats treated with a calpain inhibitor resulted in prevention of increased right ventricular systolic pressure, right ventricular hypertrophy, as well as collagen deposition and thickening of pulmonary arterioles in models of hypoxia- and monocrotaline-induced pulmonary hypertension. Additionally, inhibition of calpain in vitro blocked intracellular activation of TGF-β1, which led to attenuated Smad2/3 phosphorylation and collagen synthesis. Finally, smooth muscle cells of pulmonary arterioles from patients with pulmonary arterial hypertension showed higher levels of calpain activation and intracellular active TGF-β. Our data provide evidence that calpain mediates EGF- and PDGF-induced collagen synthesis and proliferation of pulmonary artery smooth muscle cells via an intracrine TGF-β1 pathway in pulmonary hypertension.
Figures
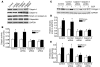


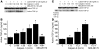
References
-
- Tuder RM. Pathology of pulmonary arterial hypertension. Semin Respir Crit Care Med. 2009;30(4):376–385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

