Phosphatase and tensin homolog (PTEN) pseudogene expression in endometrial cancer: a conserved regulatory mechanism important in tumorigenesis?
- PMID: 22005521
- PMCID: PMC3954782
- DOI: 10.1016/j.ygyno.2011.10.011
Phosphatase and tensin homolog (PTEN) pseudogene expression in endometrial cancer: a conserved regulatory mechanism important in tumorigenesis?
Abstract
Objectives: The PTEN pseudogene, PTENP1, was recently shown to play a role in cell proliferation in a prostate cancer model. In the present study, we sought to determine whether PTENP1 is expressed in endometrial cancer (EMCA) cell lines and primary tumors along with the microRNAs (miRNAs) that are predicted to regulate PTEN and PTENP1 transcript levels.
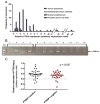
Methods: RNA was prepared from six EMCA cell lines, three normal endometrial samples, and 61 primary tumors. TaqMan® RT-PCR was used to quantitate PTEN expression in all specimens and PTENP1 expression in cell lines, and normal endometrial (NE) samples. PTENP1 expression was evaluated using conventional RT-PCR in primary tumors. MicroRNA profiling was undertaken using NanoString(TM) technology in AN3CA and KLE cell lines. The relationship between PTEN transcript levels, PTENP1 expression, and PTEN mutation status was investigated.
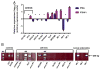
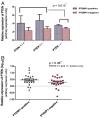
Results: All NE samples, cell lines, and primary tumors expressed PTEN. PTENP1 transcript was expressed in NE, cell lines, and 34/61 (56%) primary tumors. The median relative PTEN level was 2.9 arbitrary expression units in PTENP1-positive tumors and 2.3 in PTENP1-negative tumors (p=0.09). PTEN levels in wild-type and haploinsufficient tumors were variable compared to PTEN-null tumors (p=0.015). Four microRNAs predicted to bind PTEN/PTENP1 ranked in the top 20 most abundant microRNA subtypes in the AN3CA and KLE cell lines.
Conclusions: PTENP1 is expressed in NE and EMCA cell lines, as are PTEN/PTENP1 targeting inhibitory miRNAs (cell lines). Further studies are needed to evaluate the impact of PTEN/PTENP1/miRNA interactions on tumorigenesis regulation in EMCA.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
References
-
- ACS. Cancer facts and figures 2010. Atlanta: American Cancer Society Inc.; 2010.
-
- Ellis PE, GhaemMaghami S. Molecular characteristics and risk factors in endometrial cancer: what are the treatment and preventative strategies? Int J Gynecol Cancer. 2010;20:1207–16. - PubMed
-
- Ray M, Fleming G. Management of advanced-stage and recurrent endometrial cancer. Semin Oncol. 2009;36:145–54. - PubMed
-
- Lee NK. Adjuvant treatment of advanced-stage endometrial cancer. Clin Obstet Gynecol. 2011;54:256–65. - PubMed
-
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

