Selective silencing of Na(V)1.7 decreases excitability and conduction in vagal sensory neurons
- PMID: 22005676
- PMCID: PMC3249041
- DOI: 10.1113/jphysiol.2011.215384
Selective silencing of Na(V)1.7 decreases excitability and conduction in vagal sensory neurons
Abstract
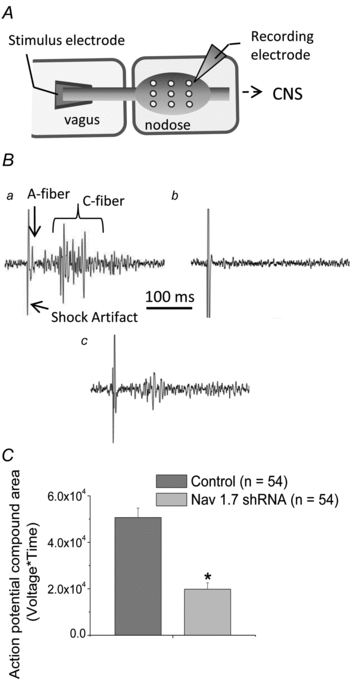
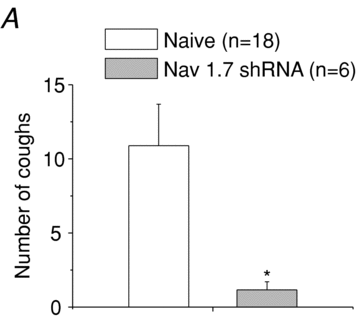
There has been much information learned in recent years about voltage gated sodium channel (Na(V)) subtypes in somatosensory pain signalling, but much less is known about the role of specific sodium channel subtypes in the vagal sensory system. In this study, we developed a technique using adeno-associated viruses (AAVs) to directly introduce shRNA against Na(V)1.7 subtype gene into the vagal sensory ganglia of guinea pigs in vivo. Na(V)1.7 gene expression in nodose ganglia was effectively and selectively reduced without influencing the expression of other sodium channel subtype genes including Na(V)1.1, 1.2, 1.3 1.6, 1.8, or 1.9. Using a whole cell patch-clamp technique, this effect on Na(V)1.7 gene expression coincided with a reduction in tetrodotoxin-sensitive sodium current, a requirement for much larger depolarizing stimulus to initiate action potentials, and reduction in repetitive action potential discharge. Extracellular recordings in the isolated vagus nerve revealed that the conduction of action potentials in sensory A- and C-fibres in many neurons was effectively abolished after Na(V)1.7 shRNA introduction. Moreover, bilateral Na(V)1.7 shRNA injected animals survived for several months and the vagal reflex behaviour, exemplified by citric acid-induced coughing, was significantly suppressed. These data indicate that selectively silencing Na(V)1.7 ion channel expression leads to a substantial decrease in neural excitability and conduction block in vagal afferent nerves.
Keywords: nav1.7.
Figures
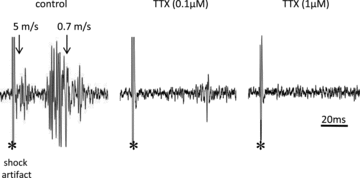
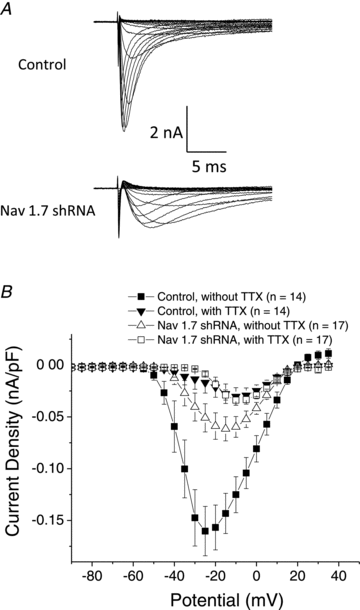

References
-
- Black JA, Renganathan M, Waxman SG. Sodium channel Nav1.6 is expressed along nonmyelinated axons and it contributes to conduction. Brain Res Mol Brain Res. 2002;105:19–28. - PubMed
-
- Caffrey JM, Eng DL, Black JA, Waxman SG, Kocsis JD. Three types of sodium channels in adult rat dorsal root ganglion neurons. Brain Res. 1992;592:283–297. - PubMed
-
- Canning BJ, Chou YL. Cough sensors. I. Physiological and pharmacological properties of the afferent nerves regulating cough. Handb Exp Pharmacol. 2009:23–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

