Analysis of pacemaker activity in the human stomach
- PMID: 22005683
- PMCID: PMC3286689
- DOI: 10.1113/jphysiol.2011.217497
Analysis of pacemaker activity in the human stomach
Abstract
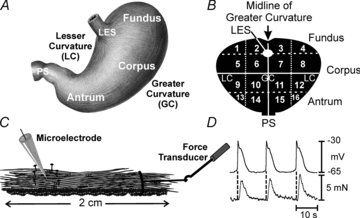
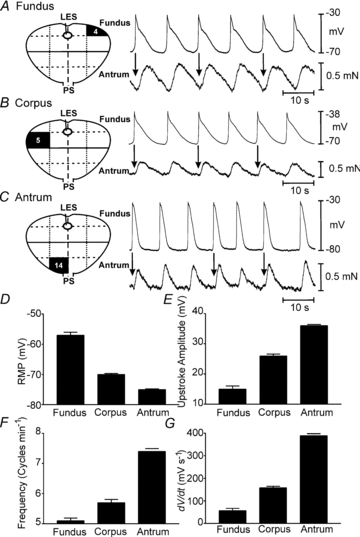
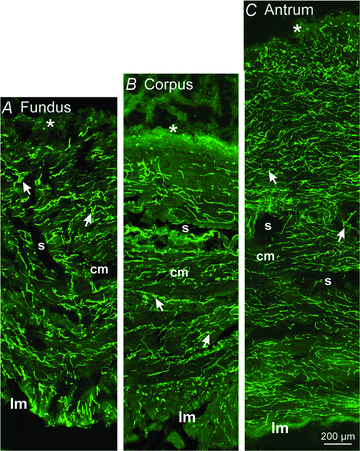
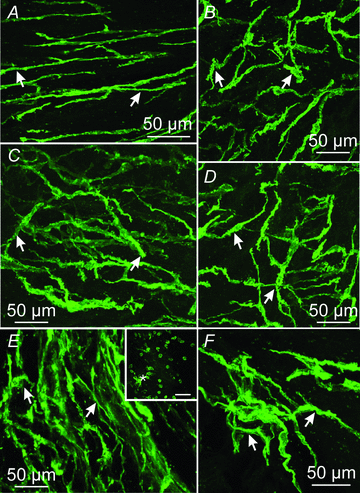
Extracellular electrical recording and studies using animal models have helped establish important concepts of human gastric physiology. Accepted standards include electrical quiescence in the fundus, 3 cycles per minute (cpm) pacemaker activity in corpus and antrum, and a proximal-to-distal slow wave frequency gradient. We investigated slow wave pacemaker activity, contractions and distribution of interstitial cells of Cajal (ICC) in human gastric muscles. Muscles were obtained from patients undergoing gastric resection for cancer, and the anatomical locations of each specimen were mapped by the operating surgeon to 16 standardized regions of the stomach. Electrical slow waves were recorded with intracellular microelectrodes and contractions were recorded by isometric force techniques. Slow waves were routinely recorded from gastric fundus muscles. These events had similar waveforms as slow waves in more distal regions and were coupled to phasic contractions. Gastric slow wave frequency was significantly greater than 3 cpm in all regions of the stomach. Antral slow wave frequency often exceeded the highest frequency of pacemaker activity in the corpus. Chronotropic mechanisms such as muscarinic and prostaglandin receptor binding, stretch, extracelluar Ca(2+) and temperature were unable to explain the observed slow wave frequency that exceeded accepted normal levels. Muscles from all regions through the thickness of the muscularis demonstrated intrinsic pacemaker activity, and this corresponded with the widespread distribution in ICC we mapped throughout the tunica muscularis. Our findings suggest that extracellular electrical recording has underestimated human slow wave frequency and mechanisms of human gastric function may differ from standard laboratory animal models.
Figures






Comment in
-
The analysis of human gastric pacemaker activity.J Physiol. 2012 Mar 1;590(5):1299-300; author reply 1301-2. doi: 10.1113/jphysiol.2011.224014. J Physiol. 2012. PMID: 22399822 Free PMC article. No abstract available.
References
-
- Carlson HC, Code CF, Nelson RA. Motor action of the canine gastroduodenal function: a cineradiographic, pressure and electric study. Am J Dig Dis. 1966;11:155–172. - PubMed
-
- Chen JDZ, Lin Z, Pan J, McCallum RW. Abnormal gastric myoelectrical activity and delayed gastric emptying in patients with symptoms suggestive of gastroparesis. Dig Dis Sci. 1996;41:1538–1545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

