In vitro pharmacodynamics of vancomycin and cefazolin alone and in combination against methicillin-resistant Staphylococcus aureus
- PMID: 22006007
- PMCID: PMC3256059
- DOI: 10.1128/AAC.05473-11
In vitro pharmacodynamics of vancomycin and cefazolin alone and in combination against methicillin-resistant Staphylococcus aureus
Abstract
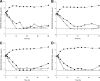
Previous studies employing time-kill methods have observed synergistic effects against methicillin-resistant Staphylococcus aureus (MRSA) when a β-lactam is combined with vancomycin. However, these time-kill studies have neglected the importance of human-simulated exposures. We evaluated the effect of human simulated exposures of vancomycin at 1 g every 8 h (q8h) in combination with cefazolin at 1 g q8h against various MRSA isolates. Four clinical isolates (two MRSA isolates [vancomycin MICs, 0.5 and 2.0 μg/ml], a heterogeneous vancomycin-intermediate S. aureus [hVISA] isolate [MIC, 2.0 μg/ml], and a vancomycin-intermediate S. aureus [VISA] isolate [MIC, 8.0 μg/ml]) were evaluated in an in vitro pharmacodynamic model with a starting inoculum of 10(6) or 10(8) CFU/ml. Bacterial density was measured over 48 to 72 h. Time-kill curves were constructed, and the area under the bacterial killing and regrowth curve (AUBC) was calculated. During 10(6) CFU/ml studies, combination therapy achieved greater log(10) CFU/ml changes than vancomycin alone at 12 h (-4.31 ± 0.58 versus -2.80 ± 0.59, P < 0.001), but not at 48 h. Combination therapy significantly reduced the AUBC from 0 to 48 h (122 ± 14) compared with vancomycin alone (148 ± 22, P = 0.017). Similar results were observed during 10(8) CFU/ml studies, where combination therapy achieved greater log(10) CFU/ml changes at 12 h than vancomycin alone (-4.00 ± 0.20 versus -1.10 ± 0.04, P < 0.001) and significantly reduced the AUBC (275 ± 30 versus 429 ± 37, P < 0.001) after 72 h of incubation. In this study, the combination of vancomycin and cefazolin at human-simulated exposures improved the rate of kill against these MRSA isolates and resulted in greater overall antibacterial effect, but no differences in bacterial density were observed by the end of the experiments.
Figures

References
-
- Albrecht LM, Rybak MJ, Warbasse LH, Edwards DJ. 1991. Vancomycin protein binding in patients with infections caused by Staphylococcus aureus. DICP 25:713–715 - PubMed
-
- Blaser J. 1985. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J. Antimicrob. Chemother. 15(Suppl A):125–130 - PubMed
-
- Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. 2004. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin. Infect. Dis. 38:448–451 - PubMed
-
- Clinical and Laboratory Standards Institute 2007. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed CLSI document M7-A7, vol 26, no 2 Clinical and Laboratory Standards Institute, Wayne, PA
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

