β-arrestin2 plays permissive roles in the inhibitory activities of RGS9-2 on G protein-coupled receptors by maintaining RGS9-2 in the open conformation
- PMID: 22006018
- PMCID: PMC3233032
- DOI: 10.1128/MCB.05690-11
β-arrestin2 plays permissive roles in the inhibitory activities of RGS9-2 on G protein-coupled receptors by maintaining RGS9-2 in the open conformation
Abstract
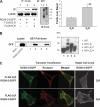
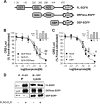
Together with G protein-coupled receptor (GPCR) kinases (GRKs) and β-arrestins, RGS proteins are the major family of molecules that control the signaling of GPCRs. The expression pattern of one of these RGS family members, RGS9-2, coincides with that of the dopamine D(3) receptor (D(3)R) in the brain, and in vivo studies have shown that RGS9-2 regulates the signaling of D2-like receptors. In this study, β-arrestin2 was found to be required for scaffolding of the intricate interactions among the dishevelled-EGL10-pleckstrin (DEP) domain of RGS9-2, Gβ5, R7-binding protein (R7BP), and D(3)R. The DEP domain of RGS9-2, under the permission of β-arrestin2, inhibited the signaling of D(3)R in collaboration with Gβ5. β-Arrestin2 competed with R7BP and Gβ5 so that RGS9-2 is placed in the cytosolic region in an open conformation which is able to inhibit the signaling of GPCRs. The affinity of the receptor protein for β-arrestin2 was a critical factor that determined the selectivity of RGS9-2 for the receptor it regulates. These results show that β-arrestins function not only as mediators of receptor-G protein uncoupling and initiators of receptor endocytosis but also as scaffolding proteins that control and coordinate the inhibitory effects of RGS proteins on the signaling of certain GPCRs.
Figures







References
-
- Ballon D. R., et al. 2006. DEP domain-mediated regulation of GPCR signaling responses. Cell 126:1079–1093 - PubMed
-
- Beom S., Cheong D., Torres G., Caron M. G., Kim K. M. 2004. Comparative studies of molecular mechanisms of dopamine D2 and D3 receptors for the activation of extracellular signal-regulated kinase. J. Biol. Chem. 279:28304–28314 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
