Global phosphoproteome profiling reveals unanticipated networks responsive to cisplatin treatment of embryonic stem cells
- PMID: 22006019
- PMCID: PMC3233030
- DOI: 10.1128/MCB.05258-11
Global phosphoproteome profiling reveals unanticipated networks responsive to cisplatin treatment of embryonic stem cells
Abstract
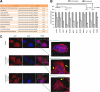
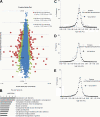
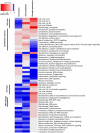
Cellular responses to DNA-damaging agents involve the activation of various DNA damage signaling and transduction pathways. Using quantitative and high-resolution tandem mass spectrometry, we determined global changes in protein level and phosphorylation site profiles following treatment of SILAC (stable isotope labeling by amino acids in cell culture)-labeled murine embryonic stem cells with the anticancer drug cisplatin. Network and pathway analyses indicated that processes related to the DNA damage response and cytoskeleton organization were significantly affected. Although the ATM (ataxia telangiectasia mutated) and ATR (ATM and Rad3-related) consensus sequence (S/T-Q motif) was significantly overrepresented among hyperphosphorylated peptides, about half of the >2-fold-upregulated phosphorylation sites based on the consensus sequence were not direct substrates of ATM and ATR. Eleven protein kinases mainly belonging to the mitogen-activated protein kinase (MAPK) family were identified as being regulated in their kinase domain activation loop. The biological importance of three of these kinases (cyclin-dependent kinase 7 [CDK7], Plk1, and KPCD1) in the protection against cisplatin-induced cytotoxicity was demonstrated by small interfering RNA (siRNA)-mediated knockdown. Our results indicate that the cellular response to cisplatin involves a variety of kinases and phosphatases not only acting in the nucleus but also regulating cytoplasmic targets, resulting in extensive cytoskeletal rearrangements. Integration of transcriptomic and proteomic data revealed a poor correlation between changes in the relative levels of transcripts and their corresponding proteins, but a large overlap in affected pathways at the levels of mRNA, protein, and phosphoprotein. This study provides an integrated view of pathways activated by genotoxic stress and deciphers kinases that play a pivotal role in regulating cellular processes other than the DNA damage response.
Figures


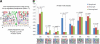
References
-
- Bartek J., Lukas J. 2007. DNA damage checkpoints: from initiation to recovery or adaptation. Curr. Opin. Cell Biol. 19:238–245 - PubMed
-
- Bensimon A., et al. 2010. ATM-dependent and -independent dynamics of the nuclear phosphoproteome after DNA damage. Sci. Signal. 3:rs3. - PubMed
-
- Borst P., Rottenberg S., Jonkers J. 2008. How do real tumors become resistant to cisplatin? Cell Cycle 7:1353–1359 - PubMed
-
- Brozovic A., Osmak M. 2007. Activation of mitogen-activated protein kinases by cisplatin and their role in cisplatin-resistance. Cancer Lett. 251:1–16 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
