Structural evidence for the rare tautomer hypothesis of spontaneous mutagenesis
- PMID: 22006298
- PMCID: PMC3203791
- DOI: 10.1073/pnas.1114496108
Structural evidence for the rare tautomer hypothesis of spontaneous mutagenesis
Abstract
Even though high-fidelity polymerases copy DNA with remarkable accuracy, some base-pair mismatches are incorporated at low frequency, leading to spontaneous mutagenesis. Using high-resolution X-ray crystallographic analysis of a DNA polymerase that catalyzes replication in crystals, we observe that a C • A mismatch can mimic the shape of cognate base pairs at the site of incorporation. This shape mimicry enables the mismatch to evade the error detection mechanisms of the polymerase, which would normally either prevent mismatch incorporation or promote its nucleolytic excision. Movement of a single proton on one of the mismatched bases alters the hydrogen-bonding pattern such that a base pair forms with an overall shape that is virtually indistinguishable from a canonical, Watson-Crick base pair in double-stranded DNA. These observations provide structural evidence for the rare tautomer hypothesis of spontaneous mutagenesis, a long-standing concept that has been difficult to demonstrate directly.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

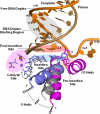

 ) captured at five different positions. λprimer and λtemplate are defined as the angle between the glycosidic bond of primer or template nucleotide and a line between the C1′ atoms of the base pair. Complete tables of all nine base-pair parameters are included (
) captured at five different positions. λprimer and λtemplate are defined as the angle between the glycosidic bond of primer or template nucleotide and a line between the C1′ atoms of the base pair. Complete tables of all nine base-pair parameters are included (

References
-
- Kunkel TA, Bebenek K. DNA replication fidelity. Annu Rev Biochem. 2000;69:497–529. - PubMed
-
- Rothwell PJ, Waksman G. Structure and mechanism of DNA polymerases. Adv Protein Chem. 2005;71:401–440. - PubMed
-
- Joyce CM, Benkovic SJ. DNA polymerase fidelity: kinetics, structure, and checkpoints. Biochemistry. 2004;43:14317–14324. - PubMed
-
- Johnson KA. Conformational coupling in DNA polymerase fidelity. Annu Rev Biochem. 1993;62:685–713. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

