Compact and flexible raster scanning multiphoton endoscope capable of imaging unstained tissue
- PMID: 22006303
- PMCID: PMC3203813
- DOI: 10.1073/pnas.1114746108
Compact and flexible raster scanning multiphoton endoscope capable of imaging unstained tissue
Abstract
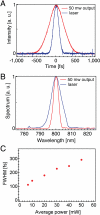
We present a compact and flexible endoscope (3-mm outer diameter, 4-cm rigid length) that utilizes a miniaturized resonant/nonresonant fiber raster scanner and a multielement gradient-index lens assembly for two-photon excited intrinsic fluorescence and second-harmonic generation imaging of biological tissues. The miniaturized raster scanner is fabricated by mounting a commercial double-clad optical fiber (DCF) onto two piezo bimorphs that are aligned such that their bending axes are perpendicular to each other. Fast lateral scanning of the laser illumination at 4.1 frames/s (512 lines per frame) is achieved by simultaneously driving the DCF cantilever at its resonant frequency in one dimension and nonresonantly in the orthogonal axis. The implementation of a DCF into the scanner enables simultaneous delivery of the femtosecond pulsed 800-nm excitation source and epi-collection of the signal. Our device is able to achieve a field-of-view (FOV(xy)) of 110 μm by 110 μm with a highly uniform pixel dwell time. The lateral and axial resolutions for two-photon imaging are 0.8 and 10 μm, respectively. The endoscope's imaging capabilities were demonstrated by imaging ex vivo mouse tissue through the collection of intrinsic fluorescence and second-harmonic signal without the need for staining. The results presented here indicate that our device can be applied in the future to perform minimally invasive in vivo optical biopsies for medical diagnostics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

