Involvement of O-glycosylation defining oncofetal fibronectin in epithelial-mesenchymal transition process
- PMID: 22006308
- PMCID: PMC3203762
- DOI: 10.1073/pnas.1115191108
Involvement of O-glycosylation defining oncofetal fibronectin in epithelial-mesenchymal transition process
Abstract
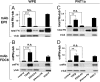
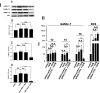
The process termed "epithelial-mesenchymal transition" (EMT) was originally discovered in ontogenic development, and has been shown to be one of the key steps in tumor cell progression and metastasis. Recently, we showed that the expression of some glycosphingolipids (GSLs) is down-regulated during EMT in human and mouse cell lines. Here, we demonstrate the involvement of GalNAc-type (or mucin-type) O-glycosylation in EMT process, induced with transforming growth factor β (TGF-β) in human prostate epithelial cell lines. We found that: (i) TGF-β treatment caused up-regulation of oncofetal fibronectin (onfFN), which is defined by mAb FDC6, and expressed in cancer or fetal cells/tissues, but not in normal adult cells/tissues. The reactivity of mAb FDC6 requires the addition of an O-glycan at a specific threonine, inside the type III homology connective segment (IIICS) domain of FN. (ii) This change is associated with typical EMT characteristics; i.e., change from epithelial to fibroblastic morphology, enhanced cell motility, decreased expression of a typical epithelial cell marker, E-cadherin, and enhanced expression of mesenchymal markers. (iii) TGF-β treatment up-regulated mRNA level of FN containing the IIICS domain and GalNAc-T activity for the IIICS domain peptide substrate containing the FDC6 onfFN epitope. (iv) Knockdown of GalNAc-T6 and T3 inhibited TGF-β-induced up-regulation of onfFN and EMT process. (v) Involvement of GSLs was not detectable with the EMT process in these cell lines. These findings indicate the important functional role of expression of onfFN, defined by site-specific O-glycosylation at IIICS domain, in the EMT process.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
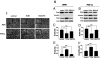


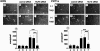
References
-
- Haltiwanger RS, Lowe JB. Role of glycosylation in development. Annu Rev Biochem. 2004;73:491–537. - PubMed
-
- Hakomori S. Traveling for the glycosphingolipid path. Glycoconj J. 2000;17:627–647. - PubMed
-
- Miljan EA, Bremer EG. Regulation of growth factor receptors by gangliosides. Science STKE. 2002;2002:11–10. RE15. - PubMed
-
- Rebbaa A, Chou PM, Bremer EG. Modulation of Growth Factor response in brain tumors by complex carbohydrates. Bull Cancer. 2004;91:E15–E60. - PubMed
-
- Todeschini AR, Dos Santos JN, Handa K, Hakomori SI. Ganglioside GM2-tetraspanin CD82 complex inhibits met and its cross-talk with integrins, providing a basis for control of cell motility through glycosynapse. J Biol Chem. 2007;282:8123–8133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

