Voltage-gated proton channel in a dinoflagellate
- PMID: 22006335
- PMCID: PMC3207696
- DOI: 10.1073/pnas.1115405108
Voltage-gated proton channel in a dinoflagellate
Abstract
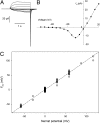
Fogel and Hastings first hypothesized the existence of voltage-gated proton channels in 1972 in bioluminescent dinoflagellates, where they were thought to trigger the flash by activating luciferase. Proton channel genes were subsequently identified in human, mouse, and Ciona intestinalis, but their existence in dinoflagellates remained unconfirmed. We identified a candidate proton channel gene from a Karlodinium veneficum cDNA library based on homology with known proton channel genes. K. veneficum is a predatory, nonbioluminescent dinoflagellate that produces toxins responsible for fish kills worldwide. Patch clamp studies on the heterologously expressed gene confirm that it codes for a genuine voltage-gated proton channel, kH(V)1: it is proton-specific and activated by depolarization, its g(H)-V relationship shifts with changes in external or internal pH, and mutation of the selectivity filter (which we identify as Asp(51)) results in loss of proton-specific conduction. Indirect evidence suggests that kH(V)1 is monomeric, unlike other proton channels. Furthermore, kH(V)1 differs from all known proton channels in activating well negative to the Nernst potential for protons, E(H). This unique voltage dependence makes the dinoflagellate proton channel ideally suited to mediate the proton influx postulated to trigger bioluminescence. In contrast to vertebrate proton channels, whose main function is acid extrusion, we propose that proton channels in dinoflagellates have fundamentally different functions of signaling and excitability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


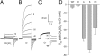
References
-
- Quatrefages A. Observations on Noctiluca. Ann Sci Natur Zool Ser. 1850;3:226–235. French.
-
- Fogel M, Schmitter RE, Hastings JW. On the physical identity of scintillons: Bioluminescent particles in Gonyaulax polyedra. J Cell Sci. 1972;11:305–317. - PubMed
-
- Chang JJ. Electrophysiological studies of a non-luminescent form of the dinoflagellate Noctiluca miliaris. J Cell Comp Physiol. 1960;56:33–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

