Measurement of human skeletal muscle oxidative capacity by 31P-MR spectroscopy: a cross-validation with in vitro measurements
- PMID: 22006551
- PMCID: PMC3201762
- DOI: 10.1002/jmri.22733
Measurement of human skeletal muscle oxidative capacity by 31P-MR spectroscopy: a cross-validation with in vitro measurements
Abstract
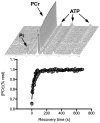
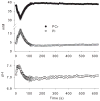
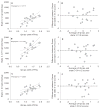
Purpose: To cross-validate skeletal muscle oxidative capacity measured by (31)P-MR spectroscopy with in vitro measurements of oxidative capacity in mitochondria isolated from muscle biopsies of the same muscle group in 18 healthy adults.
Materials and methods: Oxidative capacity in vivo was determined from PCr recovery kinetics following a 30-s maximal isometric knee extension. State 3 respiration was measured in isolated mitochondria using high-resolution respirometry. A second cohort of 10 individuals underwent two (31)P-MRS testing sessions to assess the test-retest reproducibility of the method.
Results: Overall, the in vivo and in vitro methods were well-correlated (r = 0.66-0.72) and showed good agreement by Bland Altman plots. Excellent reproducibility was observed for the PCr recovery rate constant (CV = 4.6%; ICC = 0.85) and calculated oxidative capacity (CV = 3.4%; ICC = 0.83).
Conclusion: These results indicate that (31)P-MRS corresponds well with gold-standard in vitro measurements and is highly reproducible.
Copyright © 2011 Wiley Periodicals, Inc.
Figures

Similar articles
-
A cross-validation of near-infrared spectroscopy measurements of skeletal muscle oxidative capacity with phosphorus magnetic resonance spectroscopy.J Appl Physiol (1985). 2013 Dec;115(12):1757-66. doi: 10.1152/japplphysiol.00835.2013. Epub 2013 Oct 17. J Appl Physiol (1985). 2013. PMID: 24136110 Free PMC article.
-
Effects of exercise-induced intracellular acidosis on the phosphocreatine recovery kinetics: a 31P MRS study in three muscle groups in humans.NMR Biomed. 2013 Nov;26(11):1403-11. doi: 10.1002/nbm.2966. Epub 2013 May 23. NMR Biomed. 2013. PMID: 23703831 Clinical Trial.
-
Dynamic 31P MR spectroscopy of plantar flexion: influence of ergometer design, magnetic field strength (3 and 7 T), and RF-coil design.Med Phys. 2015 Apr;42(4):1678-89. doi: 10.1118/1.4914448. Med Phys. 2015. PMID: 25832057
-
Absolute quantification of phosphorus metabolite concentrations in human muscle in vivo by 31P MRS: a quantitative review.NMR Biomed. 2007 Oct;20(6):555-65. doi: 10.1002/nbm.1192. NMR Biomed. 2007. PMID: 17628042 Review.
-
31P-MRS-Measured Phosphocreatine Recovery Kinetics in Human Muscles in Health and Disease-A Systematic Review and Meta-Analysis.NMR Biomed. 2025 May;38(5):e70023. doi: 10.1002/nbm.70023. NMR Biomed. 2025. PMID: 40189235 Review.
Cited by
-
Dynamic magnetic resonance measurements of calf muscle oxygenation and energy metabolism in peripheral artery disease.J Magn Reson Imaging. 2020 Jan;51(1):98-107. doi: 10.1002/jmri.26841. Epub 2019 Jun 19. J Magn Reson Imaging. 2020. PMID: 31218803 Free PMC article.
-
Impaired Muscle Efficiency but Preserved Peripheral Hemodynamics and Mitochondrial Function With Advancing Age: Evidence From Exercise in the Young, Old, and Oldest-Old.J Gerontol A Biol Sci Med Sci. 2018 Sep 11;73(10):1303-1312. doi: 10.1093/gerona/gly050. J Gerontol A Biol Sci Med Sci. 2018. PMID: 29584857 Free PMC article.
-
High cytochrome c oxidase expression links to severe skeletal energy failure by (31)P-MRS spectroscopy in mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes.CNS Neurosci Ther. 2014 Jun;20(6):509-14. doi: 10.1111/cns.12257. Epub 2014 Mar 27. CNS Neurosci Ther. 2014. PMID: 24674659 Free PMC article.
-
Skeletal Muscle mRNA Splicing Variants Association With Four Different Fitness and Energetic Measures in the GESTALT Study.J Cachexia Sarcopenia Muscle. 2025 Feb;16(1):e13603. doi: 10.1002/jcsm.13603. Epub 2024 Dec 2. J Cachexia Sarcopenia Muscle. 2025. PMID: 39621510 Free PMC article.
-
Effects of Dietary n-3 Fatty Acids on Hepatic and Peripheral Insulin Sensitivity in Insulin-Resistant Humans.Diabetes Care. 2015 Jul;38(7):1228-37. doi: 10.2337/dc14-3101. Epub 2015 Apr 7. Diabetes Care. 2015. PMID: 25852206 Free PMC article. Clinical Trial.
References
-
- Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242(9):2278–2282. - PubMed
-
- Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51(10):2944–2950. - PubMed
-
- Simoneau JA, Kelley DE. Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol. 1997;83(1):166–171. - PubMed
-
- Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 2007;356(11):1140–1151. - PubMed
-
- Pulkes T, Sweeney MG, Hanna MG. Increased risk of stroke in patients with the A12308G polymorphism in mitochondria. Lancet. 2000;356(9247):2068–2069. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

