Biochemical and immunologic effects of rituximab in patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid
- PMID: 22006563
- PMCID: PMC12168494
- DOI: 10.1002/hep.24748
Biochemical and immunologic effects of rituximab in patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid
Abstract
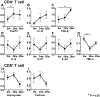
The aim of this study was to determine the safety and potential efficacy of B-cell depletion with the anti-CD20 monoclonal antibody rituximab in patients with primary biliary cirrhosis (PBC) and an incomplete response to ursodeoxycholic acid (UDCA). This open-label study enrolled six patients with PBC and incomplete responses to UDCA to be treated with 2 doses of 1000 mg rituximab separated by 2 weeks and followed for 52 weeks. The primary endpoints were safety and changes in B-cell function. Two patients received only 1 dose of rituximab, one due to activation of latent varicella and the other due to a viral upper respiratory infection. Serum levels of total IgG, IgM, and IgA as well as anti-mitochondrial autoantibodies (AMAs) IgA and IgM decreased significantly from baseline by 16 weeks and returned to baseline levels by 36 weeks. Stimulation of B cells with CpG produced significantly less IgM at 52 weeks after treatment compared with B cells at baseline. In addition, transient decreases in memory B-cell and T-cell frequencies and an increase in CD25(high) CD4(+) T cells were observed after treatment. These changes were associated with significant increases in mRNA levels of FoxP3 and transforming growth factor-β (TGF-β) and a decrease in tumor necrosis factor-α (TNF-α) in CD4(+) T cells. Notably, serum alkaline phosphatase levels were significantly reduced up to 36 weeks following rituximab treatment.
Conclusion: These data suggest that depletion of B cells influences the induction, maintenance, and activation of both B and T cells and provides a potential mechanism for treatment of patients with PBC with an incomplete response to UDCA.
Copyright © 2011 American Association for the Study of Liver Diseases.
Figures
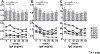





References
-
- Gershwin ME and Mackay IR. The causes of primary biliary cirrhosis: Convenient and inconvenient truths. Hepatology 2008;47:737–45. - PubMed
-
- Kaplan MM and Gershwin ME. Primary biliary cirrhosis. N Engl J Med 2005;353:1261–73. - PubMed
-
- Gershwin ME, Mackay IR, Sturgess A and Coppel RL. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol 1987;138:3525–31. - PubMed
-
- Kamihira T, Shimoda S, Harada K, Kawano A, Handa M, Baba E, et al. Distinct costimulation dependent and independent autoreactive T-cell clones in primary biliary cirrhosis. Gastroenterology 2003;125:1379–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
