Has the time come to use near-infrared spectroscopy as a routine clinical tool in preterm infants undergoing intensive care?
- PMID: 22006900
- PMCID: PMC3263787
- DOI: 10.1098/rsta.2011.0261
Has the time come to use near-infrared spectroscopy as a routine clinical tool in preterm infants undergoing intensive care?
Abstract
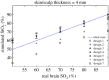
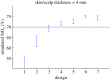
Several instruments implementing spatially resolved near-infrared spectroscopy (NIRS) to monitor tissue oxygenation are now approved for clinical use. The neonatal brain is readily assessible by NIRS and neurodevelopmental impairment is common in children who were in need of intensive care during the neonatal period. It is likely that an important part of the burden of this handicap is due to brain injury induced by hypoxia-ischaemia during intensive care. In particular, this is true for infants born extremely preterm. Thus, monitoring of cerebral oxygenation has considerable potential benefit in this group. The benefit, however, should be weighed against the disturbance to the infant, against the limitations imposed on clinical care and against costs. The ultimate way of demonstrating the 'added value' is by a randomized controlled trial. Cerebral oximetry must reduce the risk of a clinically relevant endpoint, such as death or neurodevelopmental handicap. We estimate that such a trial should recruit about 4000 infants to have the power to detect a reduction in brain injury by one-fifth. This illustrates the formidable task of providing first-grade evidence for the clinical value of diagnostic methods. Is it a window of opportunity for the establishment of a rational basis before another technology is added to an already overly complex newborn intensive care?
Figures

References
-
- Soul J. S., du Plessis A. J.1999New technologies in pediatric neurology. Near-infrared spectroscopy Semin. Pediatr. Neurol. 6101–110.10.1016/S1071-9091(99)80036-9 (doi:10.1016/S1071-9091(99)80036-9) - DOI - DOI - PubMed
-
- Wolf M., Ferrari M., Quaresima V.2007Progress of near-infrared spectroscopy and topography for brain and muscle clinical applications J. Biomed. Opt. 12062104.10.1117/1.2804899 (doi:10.1117/1.2804899) - DOI - DOI - PubMed
-
- Wolf M., Greisen G.2009Advances in near-infrared spectroscopy to study the brain of the preterm and term neonate Clin. Perinatol. 36807–834.10.1016/j.clp.2009.07.007 (doi:10.1016/j.clp.2009.07.007) - DOI - DOI - PubMed
-
- Goldman S., Sutter F., Ferdinand F., Trace C.2004Optimizing intraoperative cerebral oxygen delivery using noninvasive cerebral oximetry decreases the incidence of stroke for cardiac surgical patients Heart Surg. Forum 7E376–E381.10.1532/HSF98.20041062 (doi:10.1532/HSF98.20041062) - DOI - DOI - PubMed
-
- Murkin J., et al. 2007Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study Anesth. Analg. 10451–58.10.1213/01.ane.0000246814.29362.f4 (doi:10.1213/01.ane.0000246814.29362.f4) - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
