Implicit and explicit prior information in near-infrared spectral imaging: accuracy, quantification and diagnostic value
- PMID: 22006905
- PMCID: PMC3263784
- DOI: 10.1098/rsta.2011.0228
Implicit and explicit prior information in near-infrared spectral imaging: accuracy, quantification and diagnostic value
Abstract
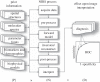
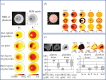
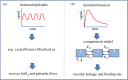
Near-infrared spectroscopy (NIRS) of tissue provides quantification of absorbers, scattering and luminescent agents in bulk tissue through the use of measurement data and assumptions. Prior knowledge can be critical about things such as (i) the tissue shape and/or structure, (ii) spectral constituents, (iii) limits on parameters, (iv) demographic or biomarker data, and (v) biophysical models of the temporal signal shapes. A general framework of NIRS imaging with prior information is presented, showing that prior information datasets could be incorporated at any step in the NIRS process, with the general workflow being: (i) data acquisition, (ii) pre-processing, (iii) forward model, (iv) inversion/reconstruction, (v) post-processing, and (vi) interpretation/diagnosis. Most of the development in NIRS has used ad hoc or empirical implementations of prior information such as pre-measured absorber or fluorophore spectra, or tissue shapes as estimated by additional imaging tools. A comprehensive analysis would examine what prior information maximizes the accuracy in recovery and value for medical diagnosis, when implemented at separate stages of the NIRS sequence. Individual applications of prior information can show increases in accuracy or improved ability to estimate biochemical features of tissue, while other approaches may not. Most beneficial inclusion of prior information has been in the inversion/reconstruction process, because it solves the mathematical intractability. However, it is not clear that this is always the most beneficial stage.
Figures




Similar articles
-
Towards the next generation of near-infrared spectroscopy.Philos Trans A Math Phys Eng Sci. 2011 Nov 28;369(1955):4425-39. doi: 10.1098/rsta.2011.0262. Philos Trans A Math Phys Eng Sci. 2011. PMID: 22006899 Review.
-
MR-Guided Near-Infrared Spectral Tomography Increases Diagnostic Performance of Breast MRI.Clin Cancer Res. 2015 Sep 1;21(17):3906-12. doi: 10.1158/1078-0432.CCR-14-2546. Epub 2015 May 27. Clin Cancer Res. 2015. PMID: 26019171 Free PMC article.
-
Accuracy limits in the determination of absolute optical properties using time-resolved NIR spectroscopy.Med Phys. 2001 Jun;28(6):1115-24. doi: 10.1118/1.1373674. Med Phys. 2001. PMID: 11439481
-
Dynamic topographical pattern classification of multichannel prefrontal NIRS signals.J Neural Eng. 2013 Aug;10(4):046018. doi: 10.1088/1741-2560/10/4/046018. Epub 2013 Jul 18. J Neural Eng. 2013. PMID: 23867792
-
[Near-infrared optical imaging of human brain function--a novel approach to the brain and the mind].Seishin Shinkeigaku Zasshi. 2002;104(5):381-93. Seishin Shinkeigaku Zasshi. 2002. PMID: 12187655 Review. Japanese.
Cited by
-
Direct Regularization From Co-Registered Contrast MRI Improves Image Quality of MRI-Guided Near-Infrared Spectral Tomography of Breast Lesions.IEEE Trans Med Imaging. 2018 May;37(5):1247-1252. doi: 10.1109/TMI.2018.2794548. IEEE Trans Med Imaging. 2018. PMID: 29727287 Free PMC article.
-
Direct regularization from co-registered anatomical images for MRI-guided near-infrared spectral tomographic image reconstruction.Biomed Opt Express. 2015 Aug 27;6(9):3618-30. doi: 10.1364/BOE.6.003618. eCollection 2015 Sep 1. Biomed Opt Express. 2015. PMID: 26417528 Free PMC article.
-
Quantification in time-domain diffuse optical tomography using Mellin-Laplace transforms.Biomed Opt Express. 2016 Sep 29;7(10):4346-4363. doi: 10.1364/BOE.7.004346. eCollection 2016 Oct 1. Biomed Opt Express. 2016. PMID: 27867736 Free PMC article.
-
Photo-magnetic imaging: resolving optical contrast at MRI resolution.Phys Med Biol. 2013 Jun 7;58(11):3551-62. doi: 10.1088/0031-9155/58/11/3551. Epub 2013 May 2. Phys Med Biol. 2013. PMID: 23640084 Free PMC article.
-
White light-informed optical properties improve ultrasound-guided fluorescence tomography of photoactive protoporphyrin IX.J Biomed Opt. 2013 Apr;18(4):046008. doi: 10.1117/1.JBO.18.4.046008. J Biomed Opt. 2013. PMID: 23584445 Free PMC article.
References
-
- Stott J. J., Culver J. P., Arridge S. R., Boas D. A.2003Optode positional calibration in diffuse optical tomography Appl. Opt. 423154–3162.10.1364/AO.42.003154 (doi:10.1364/AO.42.003154) - DOI - DOI - PubMed
-
- Schweiger M., Nissila I., Boas D. A., Arridge S. R.2007Image reconstruction in optical tomography in the presence of coupling errors Appl. Opt. 462743–2756.10.1364/AO.46.002743 (doi:10.1364/AO.46.002743) - DOI - DOI - PubMed
-
- Pogue B., McBride T., Osterberg U., Paulsen K.1999Comparison of imaging geometries for diffuse optical tomography of tissue Opt. Express 4270–286.10.1364/OE.4.000270 (doi:10.1364/OE.4.000270) - DOI - DOI - PubMed
-
- Pogue B. W., Song X., Tosteson T. D., McBride T. O., Jiang S., Paulsen K. D.2002Statistical analysis of nonlinearly reconstructed near-infrared tomographic images. I. Theory and simulations IEEE Trans. Med. Imaging 21755–763.10.1109/TMI.2002.801155 (doi:10.1109/TMI.2002.801155) - DOI - DOI - PubMed
-
- Song X., Pogue B. W., Tosteson T. D., McBride T. O., Jiang S., Paulsen K. D.2002Statistical analysis of nonlinearly reconstructed near-infrared tomographic images. II. Experimental interpretation IEEE Trans. Med. Imaging 21764–772.10.1109/TMI.2002.801158 (doi:10.1109/TMI.2002.801158) - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

