High-resolution imaging reveals indirect coordination of opposite motors and a role for LIS1 in high-load axonal transport
- PMID: 22006948
- PMCID: PMC3198168
- DOI: 10.1083/jcb.201104076
High-resolution imaging reveals indirect coordination of opposite motors and a role for LIS1 in high-load axonal transport
Abstract
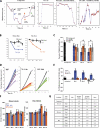
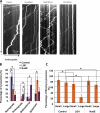
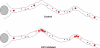
The specific physiological roles of dynein regulatory factors remain poorly understood as a result of their functional complexity and the interdependence of dynein and kinesin motor activities. We used a novel approach to overcome these challenges, combining acute in vivo inhibition with automated high temporal and spatial resolution particle tracking. Acute dynein inhibition in nonneuronal cells caused an immediate dispersal of diverse forms of cargo, resulting from a sharp decrease in microtubule minus-end run length followed by a gradual decrease in plus-end runs. Acute LIS1 inhibition or LIS1 RNA interference had little effect on lysosomes/late endosomes but severely inhibited axonal transport of large, but not small, vesicular structures. Our acute inhibition results argue against direct mechanical activation of opposite-directed motors and offer a novel approach of potential broad utility in the study of motor protein function in vivo. Our data also reveal a specific but cell type-restricted role for LIS1 in large vesicular transport and provide the first quantitative support for a general role for LIS1 in high-load dynein functions.
Figures