DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice
- PMID: 22006977
- PMCID: PMC3201196
- DOI: 10.1084/jem.20110345
DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice
Abstract
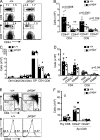
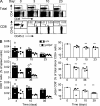
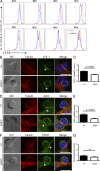
In humans, DOCK8 immunodeficiency syndrome is characterized by severe cutaneous viral infections. Thus, CD8 T cell function may be compromised in the absence of DOCK8. In this study, by analyzing mutant mice and humans, we demonstrate a critical, intrinsic role for DOCK8 in peripheral CD8 T cell survival and function. DOCK8 mutation selectively diminished the abundance of circulating naive CD8 T cells in both species, and in DOCK8-deficient humans, most CD8 T cells displayed an exhausted CD45RA(+)CCR7(-) phenotype. Analyses in mice revealed the CD8 T cell abnormalities to be cell autonomous and primarily postthymic. DOCK8 mutant naive CD8 T cells had a shorter lifespan and, upon encounter with antigen on dendritic cells, exhibited poor LFA-1 synaptic polarization and a delay in the first cell division. Although DOCK8 mutant T cells underwent near-normal primary clonal expansion after primary infection with recombinant influenza virus in vivo, they showed greatly reduced memory cell persistence and recall. These findings highlight a key role for DOCK8 in the survival and function of human and mouse CD8 T cells.
Figures





References
-
- Appay V., van Lier R.A.W., Sallusto F., Roederer M. 2008. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 73:975–983 - PubMed
-
- Atherton D.J., Gennery A.R., Cant A.J. 2004. The neonate. Rook’s Textbook of Dermatology, Vol. 1. Seventh edition Burns T., Breathnach S., Cox N., Griffiths C., Blackwell Publishing, Carlton, Australia: 14:66–14:67
-
- Badour K., Zhang J., Shi F., McGavin M.K., Rampersad V., Hardy L.A., Field D., Siminovitch K.A. 2003. The Wiskott-Aldrich syndrome protein acts downstream of CD2 and the CD2AP and PSTPIP1 adaptors to promote formation of the immunological synapse. Immunity. 18:141–154 10.1016/S1074-7613(02)00516-2 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

