Protein kinase A acts at the basal body of the primary cilium to prevent Gli2 activation and ventralization of the mouse neural tube
- PMID: 22007132
- PMCID: PMC3201661
- DOI: 10.1242/dev.070805
Protein kinase A acts at the basal body of the primary cilium to prevent Gli2 activation and ventralization of the mouse neural tube
Abstract
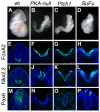
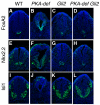
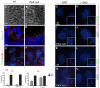
Protein kinase A (PKA) is an evolutionarily conserved negative regulator of the hedgehog (Hh) signal transduction pathway. PKA is known to be required for the proteolytic processing event that generates the repressor forms of the Ci and Gli transcription factors that keep target genes off in the absence of Hh. Here, we show that complete loss of PKA activity in the mouse leads to midgestation lethality and a completely ventralized neural tube, demonstrating that PKA is as strong a negative regulator of the sonic hedgehog (Shh) pathway as patched 1 (Ptch1) or suppressor of fused (Sufu). Genetic analysis shows that although PKA is important for production of the repressor form of Gli3, the principal function of PKA in the Shh pathway in neural development is to restrain activation of Gli2. Activation of the Hh pathway in PKA mutants depends on cilia, and the catalytic and regulatory subunits of PKA are localized to a compartment at the base of the primary cilia, just proximal to the basal body. The data show that PKA does not affect cilia length or trafficking of smoothened (Smo) in the cilium. Instead, we find that there is a significant increase in the level of Gli2 at the tips of cilia of PKA-null cells. The data suggest a model in which PKA acts at the base of the cilium after Gli proteins have transited the primary cilium; in this model the sequential movement of Gli proteins between compartments in the cilium and at its base controls accessibility of Gli proteins to PKA, which determines the fates of Gli proteins and the activity of the Shh pathway.
Figures




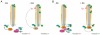
References
-
- Apionishev S., Katanayeva N. M., Marks S. A., Kalderon D., Tomlinson A. (2005). Drosophila Smoothened phosphorylation sites essential for Hedgehog signal transduction. Nat. Cell Biol. 7, 86–92 - PubMed
-
- Bai C. B., Stephen D., Joyner A. L. (2004). All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev. Cell 6, 103–115 - PubMed
-
- Barzi M., Berenguer J., Menendez A., Alvarez-Rodriguez R., Pons S. (2010). Sonic-hedgehog-mediated proliferation requires the localization of PKA to the cilium base. J. Cell Sci. 123, 62–69 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

