The dystrophin-glycoprotein complex in the prevention of muscle damage
- PMID: 22007139
- PMCID: PMC3189583
- DOI: 10.1155/2011/210797
The dystrophin-glycoprotein complex in the prevention of muscle damage
Abstract
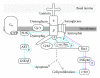
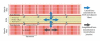
Muscular dystrophies are genetically diverse but share common phenotypic features of muscle weakness, degeneration, and progressive decline in muscle function. Previous work has focused on understanding how disruptions in the dystrophin-glycoprotein complex result in muscular dystrophy, supporting a hypothesis that the muscle sarcolemma is fragile and susceptible to contraction-induced injury in multiple forms of dystrophy. Although benign in healthy muscle, contractions in dystrophic muscle may contribute to a higher degree of muscle damage which eventually overwhelms muscle regeneration capacity. While increased susceptibility of muscle to mechanical injury is thought to be an important contributor to disease pathology, it is becoming clear that not all DGC-associated diseases share this supposed hallmark feature. This paper outlines experimental support for a function of the DGC in preventing muscle damage and examines the evidence that supports novel functions for this complex in muscle that when impaired, may contribute to the pathogenesis of muscular dystrophy.
Figures

References
-
- Wallace GQ, McNally EM. Mechanisms of muscle degeneration, regeneration, and repair in the muscular dystrophies. Annual Review of Physiology. 2009;71:37–57. - PubMed
-
- McNally EM, Pytel P. Muscle diseases: the muscular dystrophies. Annual Review of Pathology. 2007;2:87–109. - PubMed
-
- Cohn RD, Campbell KP. Molecular basis of muscular dystrophies. Muscle and Nerve. 2000;23(10):1456–1471. - PubMed
-
- Petrof BJ. The molecular basis of activity-induced muscle injury in Duchenne muscular dystrophy. Molecular and Cellular Biochemistry. 1998;179(1-2):111–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

