Dynamics of Circadian Thalamocortical Flow of Information during a Peripheral Neuropathic Pain Condition
- PMID: 22007162
- PMCID: PMC3188809
- DOI: 10.3389/fnint.2011.00043
Dynamics of Circadian Thalamocortical Flow of Information during a Peripheral Neuropathic Pain Condition
Abstract
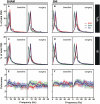
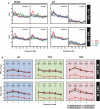
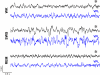
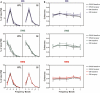
It is known that the thalamocortical loop plays a crucial role in the encoding of sensory-discriminative features of painful stimuli. However, only a few studies have addressed the changes in thalamocortical dynamics that may occur after the onset of chronic pain. Our goal was to evaluate how the induction of chronic neuropathic pain affected the flow of information within the thalamocortical loop throughout the brain states of the sleep-wake cycle. To address this issue we recorded local field potentials (LFPs) - both before and after the establishment of neuropathic pain in awake freely moving adult rats chronically implanted with arrays of multielectrodes in the lateral thalamus and primary somatosensory cortex. Our results show that the neuropathic injury induced changes in the number of wake and slow-wave-sleep (SWS) state episodes, and especially in the total number of transitions between brain states. Moreover, partial directed coherence - analysis revealed that the amount of information flow between cortex and thalamus in neuropathic animals decreased significantly, indicating that the overall thalamic activity had less weight over the cortical activity. However, thalamocortical LFPs displayed higher phase-locking during awake and SWS episodes after the nerve lesion, suggesting faster transmission of relevant information along the thalamocortical loop. The observed changes are in agreement with the hypothesis of thalamic dysfunction after the onset of chronic pain, and may result from diminished inhibitory effect of the primary somatosensory cortex over the lateral thalamus.
Keywords: intracranial recordings; partial directed coherence; rat; spared nerve injury model; thalamocortical.
Figures




Similar articles
-
Chronic Neuropathic Pain: It's about the Rhythm.J Neurosci. 2016 Jan 20;36(3):1008-18. doi: 10.1523/JNEUROSCI.2768-15.2016. J Neurosci. 2016. PMID: 26791228 Free PMC article.
-
Cortical theta is increased while thalamocortical coherence is decreased in rat models of acute and chronic pain.Pain. 2014 Apr;155(4):773-782. doi: 10.1016/j.pain.2014.01.013. Epub 2014 Jan 20. Pain. 2014. PMID: 24457192
-
Differential Excitation of Distally versus Proximally Targeting Cortical Interneurons by Unitary Thalamocortical Bursts.J Neurosci. 2016 Jun 29;36(26):6906-16. doi: 10.1523/JNEUROSCI.0739-16.2016. J Neurosci. 2016. PMID: 27358449 Free PMC article.
-
Novel neuronal and astrocytic mechanisms in thalamocortical loop dynamics.Philos Trans R Soc Lond B Biol Sci. 2002 Dec 29;357(1428):1675-93. doi: 10.1098/rstb.2002.1155. Philos Trans R Soc Lond B Biol Sci. 2002. PMID: 12626003 Free PMC article. Review.
-
Thalamic reticular nucleus in the thalamocortical loop.Neurosci Res. 2020 Jul;156:32-40. doi: 10.1016/j.neures.2019.12.004. Epub 2019 Dec 5. Neurosci Res. 2020. PMID: 31812650 Review.
Cited by
-
Circadian rhythms and pain.Neurosci Biobehav Rev. 2021 Oct;129:296-306. doi: 10.1016/j.neubiorev.2021.08.004. Epub 2021 Aug 8. Neurosci Biobehav Rev. 2021. PMID: 34375675 Free PMC article. Review.
-
Activation of dopaminergic D2/D3 receptors modulates dorsoventral connectivity in the hippocampus and reverses the impairment of working memory after nerve injury.J Neurosci. 2014 Apr 23;34(17):5861-73. doi: 10.1523/JNEUROSCI.0021-14.2014. J Neurosci. 2014. PMID: 24760846 Free PMC article.
-
Clinically Effective Treatment of Fibromyalgia Pain With High-Definition Transcranial Direct Current Stimulation: Phase II Open-Label Dose Optimization.J Pain. 2016 Jan;17(1):14-26. doi: 10.1016/j.jpain.2015.09.009. Epub 2015 Oct 9. J Pain. 2016. PMID: 26456677 Free PMC article. Clinical Trial.
-
Impaired spatial memory performance in a rat model of neuropathic pain is associated with reduced hippocampus-prefrontal cortex connectivity.J Neurosci. 2013 Feb 6;33(6):2465-80. doi: 10.1523/JNEUROSCI.5197-12.2013. J Neurosci. 2013. PMID: 23392675 Free PMC article.
-
Neuromodulation of Dopamine D2 Receptors Alters Orbitofrontal Neuronal Activity and Reduces Risk-Prone Behavior in Male Rats with Inflammatory Pain.Mol Neurobiol. 2025 Jul;62(7):8187-8203. doi: 10.1007/s12035-025-04781-0. Epub 2025 Feb 22. Mol Neurobiol. 2025. PMID: 39985709 Free PMC article.
References
LinkOut - more resources
Full Text Sources

