Rhein induces apoptosis in human breast cancer cells
- PMID: 22007260
- PMCID: PMC3189565
- DOI: 10.1155/2012/952504
Rhein induces apoptosis in human breast cancer cells
Abstract
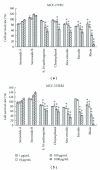
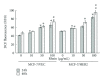
Human breast cancers cells overexpressing HER2/neu are more aggressive tumors with poor prognosis, and resistance to chemotherapy. This study investigates antiproliferation effects of anthraquinone derivatives of rhubarb root on human breast cancer cells. Of 7 anthraquinone derivatives, only rhein showed antiproliferative and apoptotic effects on both HER2-overexpressing MCF-7 (MCF-7/HER2) and control vector MCF-7 (MCF-7/VEC) cells. Rhein induced dose- and time-dependent manners increase in caspase-9-mediated apoptosis correlating with activation of ROS-mediated activation of NF-κB- and p53-signaling pathways in both cell types. Therefore, this study highlighted rhein as processing anti-proliferative activity against HER2 overexpression or HER2-basal expression in breast cancer cells and playing important roles in apoptotic induction of human breast cancer cells.
Figures






Similar articles
-
Apigenin induces apoptosis via extrinsic pathway, inducing p53 and inhibiting STAT3 and NFκB signaling in HER2-overexpressing breast cancer cells.Mol Cell Biochem. 2012 Jul;366(1-2):319-34. doi: 10.1007/s11010-012-1310-2. Epub 2012 Apr 20. Mol Cell Biochem. 2012. PMID: 22527937
-
Antitumoral actions of the anti-obesity drug orlistat (XenicalTM) in breast cancer cells: blockade of cell cycle progression, promotion of apoptotic cell death and PEA3-mediated transcriptional repression of Her2/neu (erbB-2) oncogene.Ann Oncol. 2005 Aug;16(8):1253-67. doi: 10.1093/annonc/mdi239. Epub 2005 May 3. Ann Oncol. 2005. PMID: 15870086
-
FOXO3-mediated up-regulation of Bim contributes to rhein-induced cancer cell apoptosis.Apoptosis. 2015 Mar;20(3):399-409. doi: 10.1007/s10495-014-1071-3. Apoptosis. 2015. PMID: 25501496
-
Research Progress on the Antitumor Effects of Rhein: Literature Review.Anticancer Agents Med Chem. 2017;17(12):1624-1632. doi: 10.2174/1871520615666150930112631. Anticancer Agents Med Chem. 2017. PMID: 26419468 Review.
-
HER2/neu antisense targeting of human breast carcinoma.Oncogene. 2000 Dec 11;19(53):6138-43. doi: 10.1038/sj.onc.1204001. Oncogene. 2000. PMID: 11156527 Review.
Cited by
-
Small-molecule and peptide inhibitors of m6A regulators.Front Oncol. 2025 Aug 1;15:1629864. doi: 10.3389/fonc.2025.1629864. eCollection 2025. Front Oncol. 2025. PMID: 40823087 Free PMC article. Review.
-
A Comprehensive and System Review for the Pharmacological Mechanism of Action of Rhein, an Active Anthraquinone Ingredient.Front Pharmacol. 2016 Aug 17;7:247. doi: 10.3389/fphar.2016.00247. eCollection 2016. Front Pharmacol. 2016. PMID: 27582705 Free PMC article. Review.
-
Overexpression of HER-2/neu protein attenuates the oxidative systemic profile in women diagnosed with breast cancer.Tumour Biol. 2014 Apr;35(4):3025-34. doi: 10.1007/s13277-013-1391-x. Epub 2013 Nov 19. Tumour Biol. 2014. PMID: 24248545
-
Fluorescent Rhein-Loaded Liposomes for In Vivo Biodistribution Study.Pharmaceutics. 2025 Feb 27;17(3):307. doi: 10.3390/pharmaceutics17030307. Pharmaceutics. 2025. PMID: 40142971 Free PMC article.
-
From Nature to Nanomedicine: Enhancing the Antitumor Efficacy of Rhein, Curcumin, and Resveratrol.Medicina (Kaunas). 2025 May 26;61(6):981. doi: 10.3390/medicina61060981. Medicina (Kaunas). 2025. PMID: 40572668 Free PMC article. Review.
References
-
- Huang Q, Lu G, Shen HM, Chung MC, Ong CN. Anti-cancer properties of anthraquinones from rhubarb. Medicinal Research Reviews. 2007;27(5):609–630. - PubMed
-
- Lai GH, Zhang Z, Sirica AE. Celecoxib acts in a cyclooxygenase-2-independent manner and in synergy with emodin to suppress rat cholangiocarcinoma growth in vitro through a mechanism involving enhanced Akt inactivation and increased activation of caspases-9 and -3. Molecular Cancer Therapeutics. 2003;2(3):265–271. - PubMed
-
- Yang J, Li H, Chen YY, et al. Anthraquinones sensitize tumor cells to arsenic cytotoxicity in vitro and in vivo via reactive oxygen species-mediated dual regulation of apoptosis. Free Radical Biology and Medicine. 2004;37(12):2027–2041. - PubMed
-
- Wang R, Wan Q, Zhang Y, et al. Emodin suppresses interleukin-1β induced mesangial cells proliferation and extracellular matrix production via inhibiting P38 MAPK. Life Sciences. 2007;80(26):2481–2488. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

