Electrochemical biosensors for on-chip detection of oxidative stress from immune cells
- PMID: 22007269
- PMCID: PMC3194789
- DOI: 10.1063/1.3624739
Electrochemical biosensors for on-chip detection of oxidative stress from immune cells
Abstract
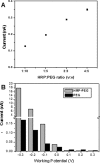
Seamless integration of biological components with electrochemical sensors is critical in the development of microdevices for cell analysis. The present paper describes the integration miniature Au electrodes next to immune cells (macrophages) in order to detect cell-secreted hydrogen peroxide (H(2)O(2)). Photopatterning of poly(ethylene glycol) (PEG) hydrogels was used to both immobilize horseradish peroxidase molecules onto electrodes and to define regions for cell attachment in the vicinity of sensing electrodes. Electrodes micropatterned in such a manner were enclosed inside poly(dimethylsiloxane) fluid conduits and incubated with macrophages. The cells attached onto the exposed glass regions in the vicinity of the electrodes and nowhere else on the non-fouling PEG hydrogel surface. A microfluidic device was converted into an electrochemical cell by placing flow-through Ag∕AgCl reference and Pt wire counter electrodes at the outlet and inlet, respectively. This microdevice with integrated H(2)O(2)-sensing electrodes had sensitivity of 27 μA∕cm(2) mM with a limit of detection of 2 μM. Importantly, this microdevice allowed controllable seeding of macrophages next to electrodes, activation of these cells and on-chip monitoring of H(2)O(2) release in real time. In the future, this biosensor platform may be utilized for monitoring of macrophage responses to pathogens or for the study of inflammatory signaling in micropatterned cell cultures.
Figures




Similar articles
-
Micropatterned surfaces functionalized with electroactive peptides for detecting protease release from cells.Anal Chem. 2013 Jan 2;85(1):220-7. doi: 10.1021/ac302547p. Epub 2012 Dec 14. Anal Chem. 2013. PMID: 23181468 Free PMC article.
-
Micropatterned aptasensors for continuous monitoring of cytokine release from human leukocytes.Anal Chem. 2011 Nov 1;83(21):8286-92. doi: 10.1021/ac202117g. Epub 2011 Oct 13. Anal Chem. 2011. PMID: 21942846 Free PMC article.
-
The use of glass substrates with bi-functional silanes for designing micropatterned cell-secreted cytokine immunoassays.Biomaterials. 2011 Aug;32(23):5478-88. doi: 10.1016/j.biomaterials.2011.04.026. Epub 2011 May 7. Biomaterials. 2011. PMID: 21550110 Free PMC article.
-
Enzyme-containing hydrogel micropatterns serving a dual purpose of cell sequestration and metabolite detection.Biosens Bioelectron. 2009 Apr 15;24(8):2604-10. doi: 10.1016/j.bios.2009.01.029. Epub 2009 Jan 31. Biosens Bioelectron. 2009. PMID: 19251408 Free PMC article.
-
Miniature enzyme-based electrodes for detection of hydrogen peroxide release from alcohol-injured hepatocytes.Anal Chem. 2013 Jan 15;85(2):932-9. doi: 10.1021/ac3025619. Epub 2012 Dec 19. Anal Chem. 2013. PMID: 23163580
Cited by
-
Biosensors for immune cell analysis-A perspective.Biomicrofluidics. 2012 Jun;6(2):21301-2130113. doi: 10.1063/1.4706845. Epub 2012 Apr 26. Biomicrofluidics. 2012. PMID: 22655003 Free PMC article.
-
Preface to Special Topic: Microsystems for manipulation and analysis of living cells.Biomicrofluidics. 2011 Sep;5(3):31901-319013. doi: 10.1063/1.3641860. Epub 2011 Sep 20. Biomicrofluidics. 2011. PMID: 22007265 Free PMC article.
-
Hydrogel Based Sensors for Biomedical Applications: An Updated Review.Polymers (Basel). 2017 Aug 16;9(8):364. doi: 10.3390/polym9080364. Polymers (Basel). 2017. PMID: 30971040 Free PMC article. Review.
-
Micropatterned surfaces functionalized with electroactive peptides for detecting protease release from cells.Anal Chem. 2013 Jan 2;85(1):220-7. doi: 10.1021/ac302547p. Epub 2012 Dec 14. Anal Chem. 2013. PMID: 23181468 Free PMC article.
-
Micropatterned sensing hydrogels integrated with reconfigurable microfluidics for detecting protease release from cells.Anal Chem. 2013 Dec 17;85(24):11893-901. doi: 10.1021/ac402660z. Epub 2013 Nov 26. Anal Chem. 2013. PMID: 24255999 Free PMC article.
References
-
- Kuby J., Immunology (W.H. Freeman and Co., New York, 1998).
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
