Production of Proteolytic Enzymes by a Keratin-Degrading Aspergillus niger
- PMID: 22007293
- PMCID: PMC3191812
- DOI: 10.4061/2011/487093
Production of Proteolytic Enzymes by a Keratin-Degrading Aspergillus niger
Abstract
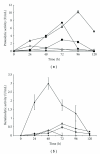
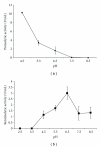
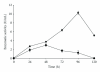
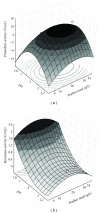
A fungal isolate with capability to grow in keratinous substrate as only source of carbon and nitrogen was identified as Aspergillus niger using the sequencing of the ITS region of the rDNA. This strain produced a slightly acid keratinase and an acid protease during cultivation in feather meal. The peak of keratinolytic activity occurred in 48 h and the maximum proteolytic activity in 96 h. These enzymes were partly characterized as serine protease and aspartic protease, respectively. The effects of feather meal concentration and initial pH on enzyme production were evaluated using a central composite design combined with response surface methodology. The optimal conditions were determined as pH 5.0 for protease and 7.8 for keratinase and 20 g/L of feather meal, showing that both models were predictive. Production of keratinases by A. niger is a less-exploited field that might represent a novel and promising biotechnological application for this microorganism.
Figures

References
-
- Roth AHFJ, Dersch P. A novel expression system for intracellular production and purification of recombinant affinity-tagged proteins in Aspergillus niger . Applied Microbiology and Biotechnology. 2010;86(2):659–670. - PubMed
-
- Schuster E, Dunn-Coleman N, Frisvad J, van Dijck P. On the safety of Aspergillus niger—a review. Applied Microbiology and Biotechnology. 2002;59(4-5):426–435. - PubMed
-
- van Dijck PWM, Selten GCM, Hempenius RA. On the safety of a new generation of DSM Aspergillus niger enzyme production strains. Regulatory Toxicology and Pharmacology. 2003;38(1):27–35. - PubMed
-
- Jarai G, Buxton F. Nitrogen, carbon, and pH regulation of extracellular acidic proteases of Aspergillus niger . Current Genetics. 1994;26(3):238–244. - PubMed
-
- Vishwanatha KS, Appu Rao AG, Singh SA. Characterisation of acid protease expressed from Aspergillus oryzae MTCC 5341. Food Chemistry. 2009;114(2):402–407.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

