Interaction analysis between genetic polymorphisms and pharmacodynamic effect in patients treated with adjunctive cilostazol to dual antiplatelet therapy: results of the ACCEL-TRIPLE (Accelerated Platelet Inhibition by Triple Antiplatelet Therapy According to Gene Polymorphism) study
- PMID: 22007612
- PMCID: PMC3376439
- DOI: 10.1111/j.1365-2125.2011.04131.x
Interaction analysis between genetic polymorphisms and pharmacodynamic effect in patients treated with adjunctive cilostazol to dual antiplatelet therapy: results of the ACCEL-TRIPLE (Accelerated Platelet Inhibition by Triple Antiplatelet Therapy According to Gene Polymorphism) study
Abstract
What is already known about this subject: Compared with standard dual antiplatelet therapy, adjunctive cilostazol to dual antiplatelet therapy ('triple antiplatelet therapy') has a potential to reduce ischemic event occurrence after percutaneous coronary intervention. The pharmacokinetic and pharmacodynamic effects of clopidogrel have been significantly influenced by the enzyme activity of the ABCB1 C3435T and the CYP2C19 system. • For the pharmacokinetics of cilostazol, genetic polymorphisms of the CYP3A5 and CYP2C19 have been associated with the substantial interindividual variability in healthy volunteers.
What this study adds: Loss-of-function polymorphism of the CYP2C19 gene, but not the ABCB1 C3435T and CYP3A5*3 genes, affects the antiplatelet effect of triple antiplatelet therapy. Most of extensive and intermediate East Asian metabolizers (0 or 1 CYP2C19 loss-of-function allele) show adequate platelet inhibition when treated with triple antiplatelet therapy after percutaneous coronary intervention. However, carriage of 2 CYP2C19 loss-of-function alleles is still associated with the risk of high platelet reactivity (defined by by 5 µM ADP-induced maximal platelet aggregation >46%), which clinical impact needs to be validated in future clinical trials. AIMS Although adjunctive cilostazol to dual antiplatelet therapy can reduce the risks of clinical events after percutaneous coronary intervention (PCI), whether genetic polymorphism can influence the pharmacodynamics of this regimen has not been evaluated.
Methods: One hundred and twenty-seven patients treated with PCI and taking triple antiplatelet therapy (≥1 month) were enrolled. Platelet reactivity was assessed by conventional aggregometry and the VerifyNow P2Y12 assay. High on-treatment platelet reactivity (HPR) was defined as 5 µm ADP-induced maximal platelet reactivity (Agg(max) ) >46%. CYP3A5*3, CYP2C19*2/*3 and ABCB1 3435C > T were genotyped.
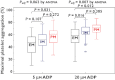
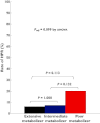
Results: CYP3A5*3 and ABCB1 3435C > T variants did not affect the antiplatelet effect of triple antiplatelet therapy. For non-carriers, one and two carriers of the CYP2C19 loss-of-function (LOF) allele, Agg(max) consecutively increased after the addition of 5 µm[mean (95% confidence intervals): 24.6% (20.8 to 28.5%) vs. 28.7% (25.4 to 32.0%) vs. 32.3% (25.8 to 38.7%), P = 0.062, respectively] and 20 µm ADP [34.2% (29.3 to 39.0%) vs. 41.7% (37.8 to 45.6%) vs. 44.9% (37.9 to 51.9%), P = 0.007, respectively]. Likewise, late platelet reactivity and P2Y12 reaction units proportionally changed according to the number of CYP2C19 LOF alleles. HPRs were observed in 9.2% of subjects: 6.3%, 7.4% and 20.0% with 0, 1 and 2 carriers of CYP2C19 LOF allele(s) (P = 0.099). In multivariate analysis, carriage of two CYP2C19 LOF alleles was a significant predictor for the prevalence of HPR (odds ratio 5.78, 95% CI 1.21, 27.78, P = 0.028).
Conclusion: Among PCI-treated patients, the effect of triple antiplatelet therapy is influenced by the CYP2C19 LOF allele. Its clinical benefit needs to be validated according to the CYP2C19 metabolic phenotype in future clinical trials. [Adjunctive Cilostazol Versus High Maintenance dose ClopidogrEL in Acute Myocardial Infarction Patients According to CYP2C19 Polymorphism (ACCEL-AMI-2C19), NCT00915733 and Adjunctive Cilostazol Versus High Maintenance-dose Clopidogrel According to Cytochrome 2C19 Polymorphism (ACCEL-2C19), NCT01012193].
© 2011 The Authors. British Journal of Clinical Pharmacology © 2011 The British Pharmacological Society.
Figures



Similar articles
-
Carriage of cytochrome 2C19 polymorphism is associated with risk of high post-treatment platelet reactivity on high maintenance-dose clopidogrel of 150 mg/day: results of the ACCEL-DOUBLE (Accelerated Platelet Inhibition by a Double Dose of Clopidogrel According to Gene Polymorphism) study.JACC Cardiovasc Interv. 2010 Jul;3(7):731-41. doi: 10.1016/j.jcin.2010.05.007. JACC Cardiovasc Interv. 2010. PMID: 20650435
-
Cytochrome 2C19 polymorphism and response to adjunctive cilostazol versus high maintenance-dose clopidogrel in patients undergoing percutaneous coronary intervention.Circ Cardiovasc Interv. 2010 Oct;3(5):450-9. doi: 10.1161/CIRCINTERVENTIONS.110.949859. Epub 2010 Sep 7. Circ Cardiovasc Interv. 2010. PMID: 20823393 Clinical Trial.
-
Platelet inhibition by adjunctive cilostazol versus high maintenance-dose clopidogrel in patients with acute myocardial infarction according to cytochrome P450 2C19 genotype.JACC Cardiovasc Interv. 2011 Apr;4(4):381-91. doi: 10.1016/j.jcin.2010.12.010. JACC Cardiovasc Interv. 2011. PMID: 21511217 Clinical Trial.
-
Comparative effects of different antiplatelet strategies in carriers of CYP2C19 loss-of-function alleles: a network meta-analysis.Eur Heart J Cardiovasc Pharmacother. 2024 Oct 4;10(6):526-536. doi: 10.1093/ehjcvp/pvae036. Eur Heart J Cardiovasc Pharmacother. 2024. PMID: 38754988
-
Comparison of on-treatment platelet reactivity between triple antiplatelet therapy with cilostazol and standard dual antiplatelet therapy in patients undergoing coronary interventions: a meta-analysis.J Cardiovasc Pharmacol Ther. 2013 Nov;18(6):533-43. doi: 10.1177/1074248413495971. Epub 2013 Jul 19. J Cardiovasc Pharmacol Ther. 2013. PMID: 23872509 Review.
Cited by
-
ABCB1 C3435T polymorphism and response to clopidogrel treatment in coronary artery disease (CAD) patients: a meta-analysis.PLoS One. 2012;7(10):e46366. doi: 10.1371/journal.pone.0046366. Epub 2012 Oct 9. PLoS One. 2012. PMID: 23056288 Free PMC article.
-
Meta-analysis of effects of ABCB1 polymorphisms on clopidogrel response among patients with coronary artery disease.Eur J Clin Pharmacol. 2017 Jul;73(7):843-854. doi: 10.1007/s00228-017-2235-1. Epub 2017 Apr 5. Eur J Clin Pharmacol. 2017. PMID: 28378058
-
CYP2C19 and ABCB1 genetic polymorphisms correlate with the recurrence of ischemic cardiovascular adverse events after clopidogrel treatment.J Clin Lab Anal. 2018 Jun;32(5):e22369. doi: 10.1002/jcla.22369. Epub 2018 Feb 4. J Clin Lab Anal. 2018. PMID: 29397568 Free PMC article.
-
Bivalirudin plus loading dose of cilostazol-based triple-antiplatelet in treatment of non-ST-elevation myocardial infarction following percutaneous coronary intervention.Ther Clin Risk Manag. 2015 Sep 28;11:1469-73. doi: 10.2147/TCRM.S86799. eCollection 2015. Ther Clin Risk Manag. 2015. PMID: 26451112 Free PMC article.
References
-
- Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, Malmberg K, Rupprecht H, Zhao F, Chrolavicius S, Copland I, Fox KA. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358:527–33. - PubMed
-
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. - PubMed
-
- Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, Wilson PW, Alberts MJ, D'Agostino R, Liau CS, Mas JL, Röther J, Smith SC, Jr, Salette G, Contant CF, Massaro JM, Steg PG REACH Registry Investigators. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–7. - PubMed
-
- Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, Bhatt DL, Cattaneo M, Collet JP, Cuisset T, Gachet C, Montalescot G, Jennings LK, Kereiakes D, Sibbing D, Trenk D, Van Werkum JW, Paganelli F, Price MJ, Waksman R, Gurbel PA Working Group on High On-Treatment Platelet Reactivity. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56:919–33. - PubMed
-
- Goto S. Cilostazol: potential mechanism of action for antithrombotic effects accompanied by a low rate of bleeding. Atheroscler Suppl. 2005;6:3–11. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

