Atp8a1 deficiency is associated with phosphatidylserine externalization in hippocampus and delayed hippocampus-dependent learning
- PMID: 22007859
- PMCID: PMC3243772
- DOI: 10.1111/j.1471-4159.2011.07543.x
Atp8a1 deficiency is associated with phosphatidylserine externalization in hippocampus and delayed hippocampus-dependent learning
Abstract
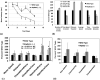
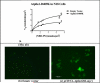
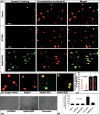
The molecule responsible for the enzyme activity plasma membrane (PM) aminophospholipid translocase (APLT), which catalyzes phosphatidylserine (PS) translocation from the outer to the inner leaflet of the plasma membrane, is unknown in mammals. A Caenorhabditis elegans study has shown that ablation of transbilayer amphipath transporter-1 (TAT-1), which is an ortholog of a mammalian P-type ATPase, Atp8a1, causes PS externalization in the germ cells. We demonstrate here that the hippocampal cells of the dentate gyrus, and Cornu Ammonis (CA1, CA3) in mice lacking Atp8a1 exhibit a dramatic increase in PS externalization. Although their hippocampi showed no abnormal morphology or heightened apoptosis, these mice displayed increased activity and a marked deficiency in hippocampus-dependent learning, but no hyper-anxiety. Such observations indicate that Atp8a1 plays a crucial role in PM-APLT activity in the neuronal cells. In corroboration, ectopic expression of Atp8a1 but not its close homolog, Atp8a2, caused an increase in the population (V(max) ) of PM-APLT without any change in its signature parameter K(m) in the neuronal N18 cells. Conversely, expression of a P-type phosphorylation-site mutant of Atp8a1 (Atp8a1*) caused a decrease in V(max) of PM-APLT without significantly altering its K(m) . The Atp8a1*-expressing N18 cells also exhibited PS externalization without apoptosis. Together, our data strongly indicate that Atp8a1 plays a central role in the PM-APLT activity of some mammalian cells, such as the neuronal N18 and hippocampal cells.
© 2011 The Authors. Journal of Neurochemistry © 2011 International Society for Neurochemistry.
Figures



References
-
- Adayev T, Estephan R, Meserole S, Mazza B, Yurkow EJ, Banerjee P. Externalization of phosphatidylserine may not be an early signal of apoptosis in neuronal cells, but only the phosphatidylserine-displaying apoptotic cells are phagocytosed by microglia. J. Neurochem. 1998;71:1854–1864. - PubMed
-
- Adayev T, Ray I, Sondhi R, Sobocki T, Banerjee P. The G protein-coupled 5-HT1A receptor causes suppression of caspase-3 through MAPK and protein kinase Cα. Biochim. Biophys. Acta. 2003;1640:85–96. - PubMed
-
- Arms K, Camp P. Biology. Harcourt Brace College Publishers; New York: 1995.
-
- Bratton DL, Hensen PM. Apoptotic Cell Recognition: Will the Real Phosphatidylserine Receptor(s) Please Stand up? Current Biology. 2007;18:R76–R79. - PubMed
-
- Bratton DL, Fadok VA, Richter DA, Kailey JM, Guthrie LA, Henson PM. Appearance of phosphatidylserine on apoptotic cells requires calcium-mediated non-specific flip-flop and is enhanced by loss of the aminophospholipid translocase. J. Biol. Chem. 1997;272:26159–26165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

