Anticholinergic versus botulinum toxin A comparison trial for the treatment of bothersome urge urinary incontinence: ABC trial
- PMID: 22008247
- PMCID: PMC3263350
- DOI: 10.1016/j.cct.2011.09.019
Anticholinergic versus botulinum toxin A comparison trial for the treatment of bothersome urge urinary incontinence: ABC trial
Abstract
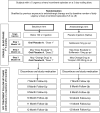
This trial compares the change in urgency urinary incontinence episodes over 6 months, tolerability and cost effectiveness between women receiving daily anticholinergic therapy plus a single intra-detrusor injection of saline versus a single intra-detrusor injection of 100 U of botulinum toxin A plus daily oral placebo tablets. We present the rationale and design of a randomized-controlled trial, Anticholinergic versus Botulinum Toxin, Comparison Trial for the Treatment of Bothersome Urge Urinary Incontinence: ABC trial, conducted by the NICHD-funded Pelvic Floor Disorders Network. We discuss the innovative nature of this trial and the challenges related to choice of patient population, maintaining masking, cost effectiveness, ethical considerations, measuring adherence, and placebo development and testing. Enrollment began in April, 2010. 242 participants will be randomized and primary outcome data analysis is anticipated to begin in mid 2012. Several challenges in the trial design are discussed. Randomization to placebo intra-detrusor injections may limit recruitment, potentially impacting generalizability. Other challenges included the heavy marketing of drugs for overactive bladder which could impact recruitment of drug-naïve women. In addition, anticholinergic medications often cause dry mouth, making masking difficult. Finally, adverse reporting of transient urinary retention is challenging as there is no standardized definition; yet this is the most common adverse event following intra-detrusor botulinum toxin injection. The ABC trial will help women with urgency urinary incontinence balance efficacy, side effects and cost of anticholinergic medication versus botulinum toxin intra-detrusor injection. The results have the potential to fundamentally change the therapeutic approach to this condition.
Trial registration: ClinicalTrials.gov NCT01166438.
Copyright © 2011 Elsevier Inc. All rights reserved.
References
-
- Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20:327–36. - PubMed
-
- Hartmann KE, McPheeters ML, Biller DH, et al. Prepared by the Vanderbilt Evidence-based Practice Center under Contract No 290-2007-10065-I. Evid Rep Technol Assess (Summ) Rockville, MD: Agency for Healthcare Research and Quality; 2009. Treatment of Overactive Bladder in Women; pp. 1–8. AHRQ Publication No 09-E017. - PMC - PubMed
-
- Diokno AC, Sand PK, Macdiarmid S, Shah R, Armstrong RB. Perceptions and behaviours of women with bladder control problems. Fam Pract. 2006;23:568–77. - PubMed
-
- Schurch B, Stohrer M, Kramer G, Schmid DM, Gaul G, Hauri D. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? Preliminary results. J Urol. 2000;164:692–7. - PubMed
-
- Sahai A, Khan MS, Dasgupta P. Efficacy of botulinum toxin-A for treating idiopathic detrusor overactivity: results from a single center, randomized, double-blind, placebo controlled trial. J Urol. 2007;177:2231–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 HD041261/HD/NICHD NIH HHS/United States
- U01 HD041249/HD/NICHD NIH HHS/United States
- U10 HD054215/HD/NICHD NIH HHS/United States
- 1U10 HD54215/HD/NICHD NIH HHS/United States
- U10 HD041267/HD/NICHD NIH HHS/United States
- 2U10 HD41250/HD/NICHD NIH HHS/United States
- U10 HD041248/HD/NICHD NIH HHS/United States
- 1U10 HD54136/HD/NICHD NIH HHS/United States
- 2U10 HD41267/HD/NICHD NIH HHS/United States
- U10 HD054241/HD/NICHD NIH HHS/United States
- U10 HD041250/HD/NICHD NIH HHS/United States
- 1U10 HD54241/HD/NICHD NIH HHS/United States
- 2U10 HD41261/HD/NICHD NIH HHS/United States
- 2U01 HD41249/HD/NICHD NIH HHS/United States
- U10 HD054214/HD/NICHD NIH HHS/United States
- 1U10 HD54214/HD/NICHD NIH HHS/United States
- UG1 HD041267/HD/NICHD NIH HHS/United States
- U10 HD054136/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous