Dexamethasone induces apoptosis in the developing rat amygdala in an age-, region-, and sex-specific manner
- PMID: 22008524
- PMCID: PMC3237835
- DOI: 10.1016/j.neuroscience.2011.09.052
Dexamethasone induces apoptosis in the developing rat amygdala in an age-, region-, and sex-specific manner
Abstract
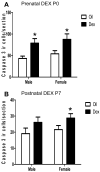
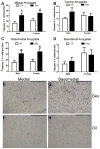
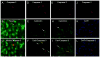
Exposure to glucocorticoids (GCs) in early development can lead to long-term changes in brain function and behavior, although little is known about the underlying neural mechanisms. Perinatal exposure to GCs alters adult anxiety and neuroendocrine responses to stress. Therefore, we investigated the effects of either late gestational or neonatal exposure to the GC receptor agonist dexamethasone (DEX), on apoptosis within the amygdala, a region critical for emotional regulation. DEX was administered to timed-pregnant rat dams from gestational day 18 until parturition, or postnatal day 4-6. Offspring were sacrificed the day following the last DEX treatment, and tissue was processed for immunohistochemical detection of cleaved caspase-3, a marker for apoptotic cells. Prenatal DEX treatment significantly increased the number of cleaved caspase-3-positive cells in the amygdala of both sexes, largely due to increases within the medial and basomedial subregions. Postnatal DEX treatment also increased cleaved caspase-3 immunoreactivity within the amygdala, although effects reached significance only in the central nucleus of females. Overall, DEX induction of cleaved caspase-3 in the amygdala was greater following prenatal compared with postnatal treatment, yet in both instances, elevations in cleaved caspase-3 correlated with an increase in pro-apoptotic Bax mRNA expression. Dual-label immunohistochemistry of cleaved caspase-3 and the neuronal marker NeuN confirmed that virtually all cleaved caspase-3-positive cells in the amygdala were neurons, and a subset of these cells (primarily following postnatal treatment) expressed a GABAergic calcium-binding protein phenotype (calbindin or calretinin). Together these results indicate that early developmental GC exposure induces neuronal apoptosis within the amygdala in an age-, sex-, and region-dependent manner.
Copyright © 2011 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Prenatal dexamethasone selectively decreases calretinin expression in the adult female lateral amygdala.Neurosci Lett. 2012 Jul 19;521(2):109-14. doi: 10.1016/j.neulet.2012.05.058. Epub 2012 Jun 2. Neurosci Lett. 2012. PMID: 22668856 Free PMC article.
-
Perinatal dexamethasone-induced alterations in apoptosis within the hippocampus and paraventricular nucleus of the hypothalamus are influenced by age and sex.J Neurosci Res. 2012 Jul;90(7):1403-12. doi: 10.1002/jnr.23026. Epub 2012 Mar 2. J Neurosci Res. 2012. PMID: 22388926
-
Sex-dependent programming effects of prenatal glucocorticoid treatment on the developing serotonin system and stress-related behaviors in adulthood.Neuroscience. 2016 Apr 21;320:43-56. doi: 10.1016/j.neuroscience.2016.01.055. Epub 2016 Feb 2. Neuroscience. 2016. PMID: 26844389 Free PMC article.
-
Acute in utero exposure to lipopolysaccharide induces inflammation in the pre- and postnatal brain and alters the glial cytoarchitecture in the developing amygdala.J Neuroinflammation. 2017 Nov 2;14(1):212. doi: 10.1186/s12974-017-0981-8. J Neuroinflammation. 2017. PMID: 29096641 Free PMC article.
-
Neurotoxicity of glucocorticoids in the primate brain.Horm Behav. 1994 Dec;28(4):336-48. doi: 10.1006/hbeh.1994.1030. Horm Behav. 1994. PMID: 7729802 Review.
Cited by
-
Exposure to dexamethasone during late gestation causes female-specific decreases in core body temperature and prepro-thyrotropin-releasing hormone expression in the paraventricular nucleus of the hypothalamus in rats.Physiol Behav. 2012 Dec 25;108:6-12. doi: 10.1016/j.physbeh.2012.07.010. Epub 2012 Aug 2. Physiol Behav. 2012. PMID: 22884559 Free PMC article.
-
Prenatal dexamethasone selectively decreases calretinin expression in the adult female lateral amygdala.Neurosci Lett. 2012 Jul 19;521(2):109-14. doi: 10.1016/j.neulet.2012.05.058. Epub 2012 Jun 2. Neurosci Lett. 2012. PMID: 22668856 Free PMC article.
-
Fetal hormonal programming of sex differences in depression: linking women's mental health with sex differences in the brain across the lifespan.Front Neurosci. 2014 Sep 8;8:247. doi: 10.3389/fnins.2014.00247. eCollection 2014. Front Neurosci. 2014. PMID: 25249929 Free PMC article. No abstract available.
-
Patterns of cell death in the perinatal mouse forebrain.J Comp Neurol. 2017 Jan 1;525(1):47-64. doi: 10.1002/cne.24041. Epub 2016 Jun 13. J Comp Neurol. 2017. PMID: 27199256 Free PMC article.
-
Sex differences in major depression and comorbidity of cardiometabolic disorders: impact of prenatal stress and immune exposures.Neuropsychopharmacology. 2019 Jan;44(1):59-70. doi: 10.1038/s41386-018-0146-1. Epub 2018 Jul 7. Neuropsychopharmacology. 2019. PMID: 30030541 Free PMC article. Review.
References
-
- Akmaev IG, Kalimullina LB, Sharipova LA. The central nucleus of the amygdaloid body of the brain: cytoarchitectonics, neuronal organization, connections. Neurosci Behav Physiol. 2004;34(6):603–10. - PubMed
-
- Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. - PubMed
-
- al Maskati HA, Zbrozyna AW. Cardiovascular and motor components of the defence reaction elicited in rats by electrical and chemical stimulation in amygdala. J Auton Nerv Syst. 1989;28(2):127–31. - PubMed
-
- Almeida OF, Condé GL, Crochemore C, Demeneix BA, Fischer D, Hassan AH, Meyer M, Holsboer F, Michaelidis TM. Subtle shifts in the ratio between pro- and antiapoptotic molecules after activation of corticosteroid receptors decide neuronal fate. FASEB J. 2000;14(5):779–90. - PubMed
-
- Arya V, Demarco VG, Issar M, Hochhaus G. Contrary to adult, neonatal rats show pronounced brain uptake of corticosteroids. Drug Metab Dispos. 2006;34(6):939–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

