Differential requirements for Myocyte Enhancer Factor-2 during adult myogenesis in Drosophila
- PMID: 22008792
- PMCID: PMC3246536
- DOI: 10.1016/j.ydbio.2011.09.031
Differential requirements for Myocyte Enhancer Factor-2 during adult myogenesis in Drosophila
Abstract
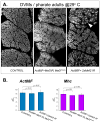
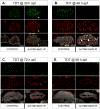
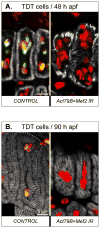
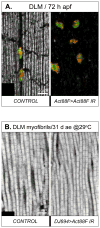
Identifying the genetic program that leads to formation of functionally and morphologically distinct muscle fibers is one of the major challenges in developmental biology. In Drosophila, the Myocyte Enhancer Factor-2 (MEF2) transcription factor is important for all types of embryonic muscle differentiation. In this study we investigated the role of MEF2 at different stages of adult skeletal muscle formation, where a diverse group of specialized muscles arises. Through stage- and tissue-specific expression of Mef2 RNAi constructs, we demonstrate that MEF2 is critical at the early stages of adult myoblast fusion: mutant myoblasts are attracted normally to their founder cell targets, but are unable to fuse to form myotubes. Interestingly, ablation of Mef2 expression at later stages of development showed MEF2 to be more dispensable for structural gene expression: after myoblast fusion, Mef2 knockdown did not interrupt expression of major structural gene transcripts, and myofibrils were formed. However, the MEF2-depleted fibers showed impaired integrity and a lack of fibrillar organization. When Mef2 RNAi was induced in muscles following eclosion, we found no adverse effects of attenuating Mef2 function. We conclude that in the context of adult myogenesis, MEF2 remains an essential factor, participating in control of myoblast fusion, and myofibrillogenesis in developing myotubes. However, MEF2 does not show a major requirement in the maintenance of muscle structural gene expression. Our findings point to the importance of a diversity of regulatory factors that are required for the formation and function of the distinct muscle fibers found in animals.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures


References
-
- Anant S, Roy S, VijayRaghavan K. Twist and Notch negatively regulate adult muscle differentiation in Drosophila. Development. 1998;125:1361–9. - PubMed
-
- Andres V, Cervera M, Mahdavi V. Determination of the consensus binding site for MEF2 expressed in muscle and brain reveals tissue-specific sequence constraints. J Biol Chem. 1995;270:23246–9. - PubMed
-
- Apitz H. pChs-Gal4, a vector for the generation of Drosophila Gal4 lines driven by identified enhancer elements. Dros Inf Serv. 2002;85:118–120.
-
- Arredondo JJ, Ferreres RM, Maroto M, Cripps RM, Marco R, Bernstein SI, Cervera M. Control of Drosophila paramyosin/miniparamyosin gene expression. Differential regulatory mechanisms for muscle-specific transcription. J Biol Chem. 2001;276:8278–87. - PubMed
-
- Atreya KB, Fernandes JJ. Founder cells regulate fiber number but not fiber formation during adult myogenesis in Drosophila. Dev Biol. 2008;321:123–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

