Rad51 promoter-targeted gene therapy is effective for in vivo visualization and treatment of cancer
- PMID: 22008909
- PMCID: PMC3277325
- DOI: 10.1038/mt.2011.215
Rad51 promoter-targeted gene therapy is effective for in vivo visualization and treatment of cancer
Abstract
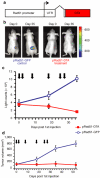
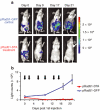
Rad51 protein is overexpressed in a wide range of human cancers. Our previous in vitro studies demonstrated that a construct comprised Rad51 promoter driving expression of the diphtheria toxin A gene (pRad51-diphtheria toxin A (DTA)) destroys a variety of human cancer cell lines, with minimal to no toxicity to normal human cells. Here we delivered Rad51 promoter-based constructs in vivo using linear polyethylenimine nanoparticles, in vivo jetPEI, to visualize and treat tumors in mice with HeLa xenografts. For tumor detection, we used pRad51-Luc, a construct containing the firefly luciferase under the Rad51 promoter, administered by intraperitoneal (IP) injection. Tumors were detected with an in vivo bioluminescent camera. All mice with cancer displayed strong bioluminescence, while mice without cancer displayed no detectable bioluminescence. Treatment with pRad51-DTA/jetPEI decreased tumor mass of subcutaneous (SC) and IP tumors by sixfold and fourfold, respectively, along with the strong reduction of malignant ascites. Fifty percent of the mice with SC tumors were cancer-free after six pRad51-DTA/jetPEI injections, and for the mice with IP tumors, mean survival time increased by 90% compared to control mice. This study demonstrates the clinical potential of pRad51-based constructs delivered by nanoparticles for the diagnostics and treatment of a wide range of cancers.
Figures





Similar articles
-
Use of the XRCC2 promoter for in vivo cancer diagnosis and therapy.Cell Death Dis. 2018 Apr 1;9(4):420. doi: 10.1038/s41419-018-0453-9. Cell Death Dis. 2018. PMID: 29549248 Free PMC article.
-
Use of the Rad51 promoter for targeted anti-cancer therapy.Proc Natl Acad Sci U S A. 2008 Dec 30;105(52):20810-5. doi: 10.1073/pnas.0807990106. Epub 2008 Dec 23. Proc Natl Acad Sci U S A. 2008. PMID: 19106292 Free PMC article.
-
Development of targeted therapy for bladder cancer mediated by a double promoter plasmid expressing diphtheria toxin under the control of H19 and IGF2-P4 regulatory sequences.J Transl Med. 2010 Dec 16;8:134. doi: 10.1186/1479-5876-8-134. J Transl Med. 2010. PMID: 21162716 Free PMC article.
-
Rad51 siRNA delivered by HVJ envelope vector enhances the anti-cancer effect of cisplatin.J Gene Med. 2005 Aug;7(8):1044-52. doi: 10.1002/jgm.753. J Gene Med. 2005. PMID: 15756713
-
Characterisation of the promoter region of the human DNA-repair gene Rad51.Eur J Gynaecol Oncol. 2005;26(6):589-98. Eur J Gynaecol Oncol. 2005. PMID: 16398215 Review.
Cited by
-
Chinese Herbal Mixture, Tien-Hsien Liquid, Induces G2/M Cycle Arrest and Radiosensitivity in MCF-7 Human Breast Cancer Cells through Mechanisms Involving DNMT1 and Rad51 Downregulation.Evid Based Complement Alternat Med. 2016;2016:3251046. doi: 10.1155/2016/3251046. Epub 2016 Jul 20. Evid Based Complement Alternat Med. 2016. PMID: 27525019 Free PMC article.
-
Promoter-Operating Targeted Expression of Gene Therapy in Cancer: Current Stage and Prospect.Mol Ther Nucleic Acids. 2018 Jun 1;11:508-514. doi: 10.1016/j.omtn.2018.04.003. Epub 2018 Apr 12. Mol Ther Nucleic Acids. 2018. PMID: 29858085 Free PMC article. Review.
-
RAD54 family translocases counter genotoxic effects of RAD51 in human tumor cells.Nucleic Acids Res. 2015 Mar 31;43(6):3180-96. doi: 10.1093/nar/gkv175. Epub 2015 Mar 12. Nucleic Acids Res. 2015. PMID: 25765654 Free PMC article.
-
A nanoparticle formulation that selectively transfects metastatic tumors in mice.Proc Natl Acad Sci U S A. 2013 Sep 3;110(36):14717-22. doi: 10.1073/pnas.1313330110. Epub 2013 Aug 19. Proc Natl Acad Sci U S A. 2013. PMID: 23959886 Free PMC article.
-
Use of a Novel Integrase-Deficient Lentivirus for Targeted Anti-Cancer Therapy With Survivin Promoter-Driven Diphtheria Toxin A.Medicine (Baltimore). 2015 Aug;94(31):e1301. doi: 10.1097/MD.0000000000001301. Medicine (Baltimore). 2015. PMID: 26252309 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

