Novel NCC mutants and functional analysis in a new cohort of patients with Gitelman syndrome
- PMID: 22009145
- PMCID: PMC3283182
- DOI: 10.1038/ejhg.2011.189
Novel NCC mutants and functional analysis in a new cohort of patients with Gitelman syndrome
Abstract
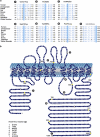
Gitelman syndrome (GS) is an autosomal recessive disorder characterized by hypokalemic metabolic alkalosis in conjunction with significant hypomagnesemia and hypocalciuria. The GS phenotype is caused by mutations in the solute carrier family 12, member 3 (SLC12A3) gene that encodes the thiazide-sensitive NaCl cotransporter (NCC). We analyzed DNA samples of 163 patients with a clinical suspicion of GS by direct sequencing of all 26 exons of the SLC12A3 gene. In total, 114 different mutations were identified, 31 of which have not been reported before. These novel variants include 3 deletions, 18 missense, 6 splice site and 4 nonsense mutations. We selected seven missense mutations to investigate their effect on NCC activity and plasma membrane localization by using the Xenopus laevis oocyte expression system. The Thr392Ile mutant did not display transport activity (probably class 2 mutation), while the Asn442Ser and Gln1030Arg NCC mutants showed decreased plasma membrane localization and consequently function, likely due to impaired trafficking (class 3 mutation). Even though the NaCl uptake was hampered for NCC mutants Glu121Asp, Pro751Leu, Ser475Cys and Tyr489His, the transporters reached the plasma membrane (class 4 mutation), suggesting an effect on NCC regulation or ion affinity. The present study shows the identification of 38 novel mutations in the SLC12A3 gene and provides insight into the mechanisms that regulate NCC.
Figures


Similar articles
-
Generation and analysis of the thiazide-sensitive Na+ -Cl- cotransporter (Ncc/Slc12a3) Ser707X knockin mouse as a model of Gitelman syndrome.Hum Mutat. 2010 Dec;31(12):1304-15. doi: 10.1002/humu.21364. Epub 2010 Oct 14. Hum Mutat. 2010. PMID: 20848653
-
Recurrent deep intronic mutations in the SLC12A3 gene responsible for Gitelman's syndrome.Clin J Am Soc Nephrol. 2011 Mar;6(3):630-9. doi: 10.2215/CJN.06730810. Epub 2010 Nov 4. Clin J Am Soc Nephrol. 2011. PMID: 21051746 Free PMC article.
-
Transcriptional and functional analyses of SLC12A3 mutations: new clues for the pathogenesis of Gitelman syndrome.J Am Soc Nephrol. 2007 Apr;18(4):1271-83. doi: 10.1681/ASN.2006101095. Epub 2007 Feb 28. J Am Soc Nephrol. 2007. PMID: 17329572
-
Gitelman syndrome.Orphanet J Rare Dis. 2008 Jul 30;3:22. doi: 10.1186/1750-1172-3-22. Orphanet J Rare Dis. 2008. PMID: 18667063 Free PMC article. Review.
-
Gitelman's syndrome: a pathophysiological and clinical update.Endocrine. 2012 Feb;41(1):53-7. doi: 10.1007/s12020-011-9556-0. Epub 2011 Nov 15. Endocrine. 2012. PMID: 22169961 Review.
Cited by
-
Gitelman syndrome with a novel frameshift variant in SLC12A3 gene accompanied by chronic kidney disease and type 2 diabetes mellitus.CEN Case Rep. 2022 May;11(2):191-195. doi: 10.1007/s13730-021-00652-4. Epub 2021 Oct 6. CEN Case Rep. 2022. PMID: 34617250 Free PMC article.
-
Gitelman syndrome with normocalciuria - a case report.BMC Nephrol. 2022 May 4;23(1):170. doi: 10.1186/s12882-022-02782-y. BMC Nephrol. 2022. PMID: 35509038 Free PMC article.
-
Functional evaluation of novel compound heterozygous variants in SLC12A3 of Gitelman syndrome.Orphanet J Rare Dis. 2025 Feb 11;20(1):66. doi: 10.1186/s13023-025-03577-8. Orphanet J Rare Dis. 2025. PMID: 39934873 Free PMC article.
-
Early onset children's Gitelman syndrome with severe hypokalaemia: a case report.BMC Pediatr. 2020 Aug 5;20(1):366. doi: 10.1186/s12887-020-02265-9. BMC Pediatr. 2020. PMID: 32758191 Free PMC article.
-
Allele-specific RT-PCR for the rapid detection of recurrent SLC12A3 mutations for Gitelman syndrome.NPJ Genom Med. 2021 Aug 13;6(1):68. doi: 10.1038/s41525-021-00230-8. NPJ Genom Med. 2021. PMID: 34389731 Free PMC article.
References
-
- Gitelman HJ, Graham JB, Welt LG. A new familial disorder characterized by hypokalemia and hypomagnesemia. Trans AssocAm Phys. 1966;79:221–235. - PubMed
-
- Melander O, Orho-Melander M, Bengtsson K, et al. Genetic variants of thiazide-sensitive NaCl-cotransporter in Gitelman's syndrome and primary hypertension. Hypertension. 2000;36:389–394. - PubMed
-
- Cruz DN, Shaer AJ, Bia MJ, Lifton RP, Simon DB. Gitelman's syndrome revisited: an evaluation of symptoms and health-related quality of life. Kidney Int. 2001;59:710–717. - PubMed
-
- Spencer RW, Voyce MA. Familial hypokalaemia and hypomagnesaemia. A further family. Acta Paediatr Scand. 1976;65:505–507. - PubMed
-
- Calo L, Punzi L, Semplicini A. Hypomagnesemia and chondrocalcinosis in Bartter's and Gitelman's syndrome: review of the pathogenetic mechanisms. Am J Nephrol. 2000;20:347–350. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

