Transglutaminase 1 and its regulator tazarotene-induced gene 3 localize to neuronal tau inclusions in tauopathies
- PMID: 22009441
- PMCID: PMC4756648
- DOI: 10.1002/path.2984
Transglutaminase 1 and its regulator tazarotene-induced gene 3 localize to neuronal tau inclusions in tauopathies
Abstract
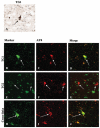
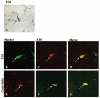
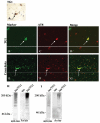
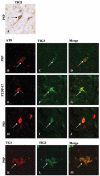
Alzheimer's disease (AD), progressive supranuclear palsy (PSP), frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17), and Pick's disease (PiD) are commonly known as tauopathies. Neurodegeneration observed in these diseases is linked to neuronal fibrillary hyperphosphorylated tau protein inclusions. Transglutaminases (TGs) are inducible enzymes, capable of modifying conformational and/or structural properties of proteins by inducing molecular cross-links. Both transglutaminase 1 (TG1) and transglutaminase 2 (TG2) are abundantly expressed in the brain and are associated with fibrillary hyperphosphorylated tau protein inclusions in neurons of AD, so-called neurofibrillary tangles (NFTs). However, other data obtained by our group suggested that tau pathology in the brain may be primarily related to TG1 and not to TG2 activity. To obtain more information on this issue, we set out to investigate the association of TG1, TG2, and TG-catalysed cross-links with fibrillary hyperphosphorylated tau inclusions in tauopathies other than AD, using immunohistochemistry. We found strong TG1 and TG-catalysed cross-link staining in neuronal tau inclusions characteristic of PSP, FTDP-17 with mutations in the tau gene (FTDP-17T), and PiD brain, whereas, in contrast to AD, TG2 was only rarely observed in these inclusions. Furthermore, using a biochemical approach, we demonstrated that tau is a substrate for TG1-mediated cross-linking. Interestingly, we found co-localization of the TG1 activator, tazarotene-induced gene 3 (TIG3), in the neuronal tau inclusions of PSP, FTDP-17T, and PiD, but not in NFTs of AD cases, indicating that these tau-containing protein aggregates are not identical. We conclude that TG1-catalysed cross-linking, regulated by TIG3, might play an important role in the formation of neuronal tau inclusions in PSP, FTDP-17T, and PiD brain.
Copyright © 2011 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
Figures

References
-
- Brion JP, Flament-Durand J, Dustin P. Alzheimer’s disease and tau proteins. Lancet. 1986;2:1098. - PubMed
-
- Grundke-Iqbal I, Iqbal K, Quinlan M, et al. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084–9. - PubMed
-
- Mandelkow EM, Mandelkow E. Tau in Alzheimer’s disease. Trends Cell Biol. 1998;8:425–7. - PubMed
-
- Nukina N, Ihara Y. One of the antigenic determinants of paired helical filaments is related to tau protein. J Biochem. 1986;99:1541–4. - PubMed
-
- Yoshida M. Cellular tau pathology and immunohistochemical study of tau isoforms in sporadic tauopathies. Neuropathology. 2006;26:457–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

