Optimized labeling of NOTA-conjugated octreotide with F-18
- PMID: 22009690
- PMCID: PMC3296034
- DOI: 10.1007/s13277-011-0250-x
Optimized labeling of NOTA-conjugated octreotide with F-18
Abstract
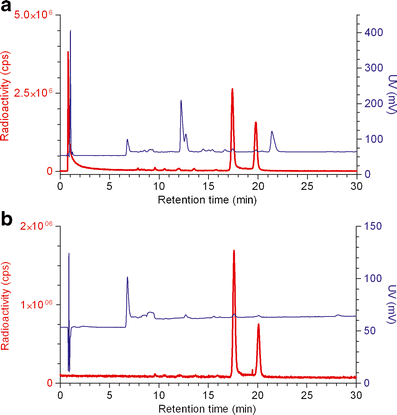
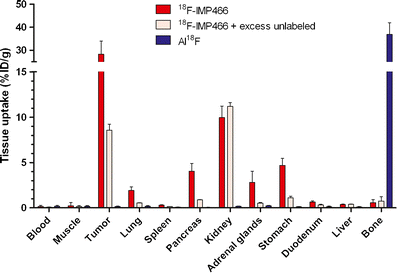
We recently reported a facile method based on the chelation of [(18)F]aluminum fluoride (Al(18)F) by NOTA (1,4,7-triazacyclononane-1,4,7-triacetic acid). Here, we present a further optimization of the (18)F labeling of NOTA-octreotide (IMP466). Octreotide was conjugated with the NOTA chelate and was labeled with (18)F in a two-step, one-pot method. The labeling procedure was optimized with regard to the labeling buffer, ionic strength, peptide concentration, and temperature. Radiochemical yield, specific activity, in vitro stability, and receptor affinity were determined. Biodistribution of (18)F-IMP466 was studied in AR42J tumor-bearing mice. In addition, microPET/CT images were acquired. IMP466 was labeled with Al(18)F in a single step with 97% yield in the presence of 80% (v/v) acetonitrile or ethanol. The labeled product was purified by HPLC to remove unlabeled peptide and unbound Al(18)F. The radiolabeling, including purification, was performed for 45 min. Specific activities of 48,000 GBq/mmol could be obtained. (18)F-IMP466 showed a high tumor uptake and excellent tumor-to-blood ratios at 2 h post-injection. In addition, the low bone uptake indicated that the Al(18)F-NOTA complex was stable in vivo. PET/CT scans revealed excellent tumor delineation and specific accumulation in the tumor. Uptake in receptor-negative organs was low. NOTA-octreotide could be labeled with (18)F in quantitative yields using a rapid two-step, one-pot, method. The compound was stable in vivo and showed rapid accretion in SSTR(2)-receptor-expressing AR42J tumors in nude mice. This method can be used to label other NOTA-conjugated compounds such as RGD peptides, GRPR-binding peptides, and Affibody molecules with (18)F.
Figures


References
-
- Poethko T, Schottelius M, Thumshirn G, Hersel U, Herz M, Henriksen G, Kessler H, Schwaiger M, Wester HJ. Two-step methodology for high-yield routine radiohalogenation of peptides: 18F-labeled RGD and octreotide analogs. J Nucl Med. 2004;45:892–902. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

