Risk of recurrence and chemotherapy benefit for patients with node-negative, estrogen receptor-positive breast cancer: recurrence score alone and integrated with pathologic and clinical factors
- PMID: 22010013
- PMCID: PMC3221521
- DOI: 10.1200/JCO.2011.35.3714
Risk of recurrence and chemotherapy benefit for patients with node-negative, estrogen receptor-positive breast cancer: recurrence score alone and integrated with pathologic and clinical factors
Abstract
Purpose: The 21-gene breast cancer assay recurrence score (RS) is widely used for assessing recurrence risk and predicting chemotherapy benefit in patients with estrogen receptor (ER) -positive breast cancer. Pathologic and clinical factors such as tumor size, grade, and patient age also provide independent prognostic utility. We developed a formal integration of these measures and evaluated its prognostic and predictive value.
Patients and methods: From the National Surgical Adjuvant Breast and Bowel (NSABP) B-14 and translational research cohort of the Arimidex, Tamoxifen Alone or in Combination (TransATAC) studies, we included patients who received hormonal monotherapy, had ER-positive tumors, and RS and traditional clinicopathologic factors assessed (647 and 1,088, respectively). Individual patient risk assessments from separate Cox models were combined using meta-analysis to form an RS-pathology-clinical (RSPC) assessment of distant recurrence risk. Risk assessments by RS and RSPC were compared in node-negative (N0) patients. RSPC was compared with RS for predicting chemotherapy benefit in NSABP B-20.
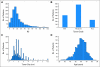
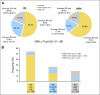
Results: RSPC had significantly more prognostic value for distant recurrence than did RS (P < .001) and showed better separation of risk in the study population. RSPC classified fewer patients as intermediate risk (17.8% v 26.7%, P < .001) and more patients as lower risk (63.8% v 54.2%, P < .001) than did RS among 1,444 N0 ER-positive patients. In B-20, the interaction of RSPC with chemotherapy was not statistically significant (P = .10), in contrast to the previously reported significant interaction of RS with chemotherapy (P = .037).
Conclusion: RSPC refines the assessment of distant recurrence risk and reduces the number of patients classified as intermediate risk. Adding clinicopathologic measures did not seem to enhance the value of RS alone nor the individual biology RS identifies in predicting chemotherapy benefit.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


Comment in
-
Anatomy and biology: two complementary sides of breast cancer prognostication.J Clin Oncol. 2011 Nov 20;29(33):4347-8. doi: 10.1200/JCO.2011.38.2754. Epub 2011 Oct 17. J Clin Oncol. 2011. PMID: 22010020 No abstract available.
References
-
- Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. - PubMed
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Breast Cancer (version 2.2008) http://www.nccn.org. - PubMed
-
- Ravdin PM, Siminoff LA, Davis GJ, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19:980–991. - PubMed
-
- Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. - PubMed
-
- Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

