Pilot induction regimen incorporating pharmacokinetically guided topotecan for treatment of newly diagnosed high-risk neuroblastoma: a Children's Oncology Group study
- PMID: 22010014
- PMCID: PMC3221519
- DOI: 10.1200/JCO.2010.34.3293
Pilot induction regimen incorporating pharmacokinetically guided topotecan for treatment of newly diagnosed high-risk neuroblastoma: a Children's Oncology Group study
Abstract
Purpose: To assess the feasibility of adding dose-intensive topotecan and cyclophosphamide to induction therapy for newly diagnosed high-risk neuroblastoma (HRNB).
Patients and methods: Enrolled patients received two cycles of topotecan (approximately 1.2 mg/m(2)/d) and cyclophosphamide (400 mg/m(2)/d) for 5 days followed by four cycles of multiagent chemotherapy (Memorial Sloan-Kettering Cancer Center [MSKCC] regimen). Pharmacokinetically guided topotecan dosing (target systemic exposure with area under the curve of 50 to 70 ng/mL/hr) was performed. Peripheral-blood stem cell (PBSC) harvest and surgical resection of residual primary tumor occurred after cycles 2 and 5, respectively. Patients achieving at least a partial response received myeloablative chemotherapy with PBSC rescue and radiation to the presurgical primary tumor volume. Oral 13-cis-retinoic acid maintenance therapy was administered twice daily for 14 days in six 28-day cycles.
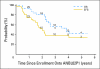
Results: Thirty-one patients were enrolled onto the study. No deaths related to toxicity or dose-limiting toxicities occurred during induction. Mucositis rarely occurred after topotecan cycles (9.7%) in contrast to 30% after MSKCC cycles. Thirty patients underwent PBSC collection with median 31.1 × 10(6) CD34+ cells/kg (range, 1.8 to 541.8 × 10(6) CD34+ cells/kg), all negative for tumor contamination by immunocytochemical analysis. Targeted topotecan systemic exposure was achieved in 26 (84%) of 31 patients. At the end of induction, 26 patients (84%) had tumor response and one patient had progressive disease. In the overall cohort, 3-year event-free and overall survival were 37.8% ± 9.4% and 57.1% ± 9.4%, respectively.
Conclusion: This pilot induction regimen was well tolerated with expected and reversible toxicities. These data support investigation of efficacy in a phase III clinical trial for newly diagnosed HRNB.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Determining the best dose for the individual patient.J Clin Oncol. 2011 Nov 20;29(33):4345-6. doi: 10.1200/JCO.2011.38.2572. Epub 2011 Oct 17. J Clin Oncol. 2011. PMID: 22010022 No abstract available.
References
-
- Park JR, Eggert A, Caron H. Neuroblastoma: Biology, prognosis, and treatment. Pediatr Clin North Am. 2008;55:97–120. - PubMed
-
- Canete A, Gerrard M, Rubie H, et al. Poor survival for infants with MYCN-amplified metastatic neuroblastoma despite intensified treatment: The International Society of Paediatric Oncology European Neuroblastoma Experience. J Clin Oncol. 2009;27:1014–1019. - PubMed
-
- Ladenstein R, Philip T, Lasset C, et al. Multivariate analysis of risk factors in stage 4 neuroblastoma patients over the age of one year treated with megatherapy and stem-cell transplantation: A report from the European Bone Marrow Transplantation Solid Tumor Registry. J Clin Oncol. 1998;16:953–965. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

